- Your cart is empty
- Continue Shopping
Biomimetic In Vitro Model for Skin Penetration and Permeability Studies
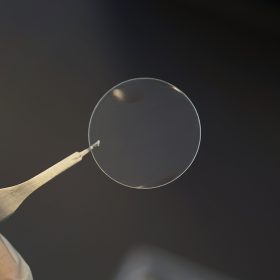
The PermeaPad® Skin Barrier is a scientifically validated biomimetic membrane developed specifically for in vitro skin penetration and permeability studies. By mimicking the structure and function of human skin, it enables researchers to accurately measure passive mass transport of drugs without the variability or ethical concerns of animal and human skin models.
Backed by validation studies from the University of Trieste, PermeaPad® has demonstrated strong correlation with human and porcine skin for compounds such as caffeine and diclofenac, making it a trusted tool for pharmaceutical and dermatology research.
The PermeaPad® Skin Barrier is a directional membrane system designed for permeability studies. It consists of two inseparable layers:
- The rough white side must face the donor compartment.
- The transparent, film-like shimmering side must face the acceptor compartment.

Key Features
-
- Biomimetic lipid membrane: Mimics human skin barrier for accurate in vitro permeability studies.
- Validated against human and porcine skin: Strong scientific correlation for drug permeation (caffeine, diclofenac, ibuprofen).
- Animal-free alternative: Ethical and reproducible replacement for biological skin models.
- Flexible and robust: Operates under multiple experimental conditions (25 °C, 37 °C).
- Easy integration: Fits standard Franz diffusion and side-by-side diffusion cells.
- Reproducible results: Enables reliable determination of permeation, flux, and recovery.
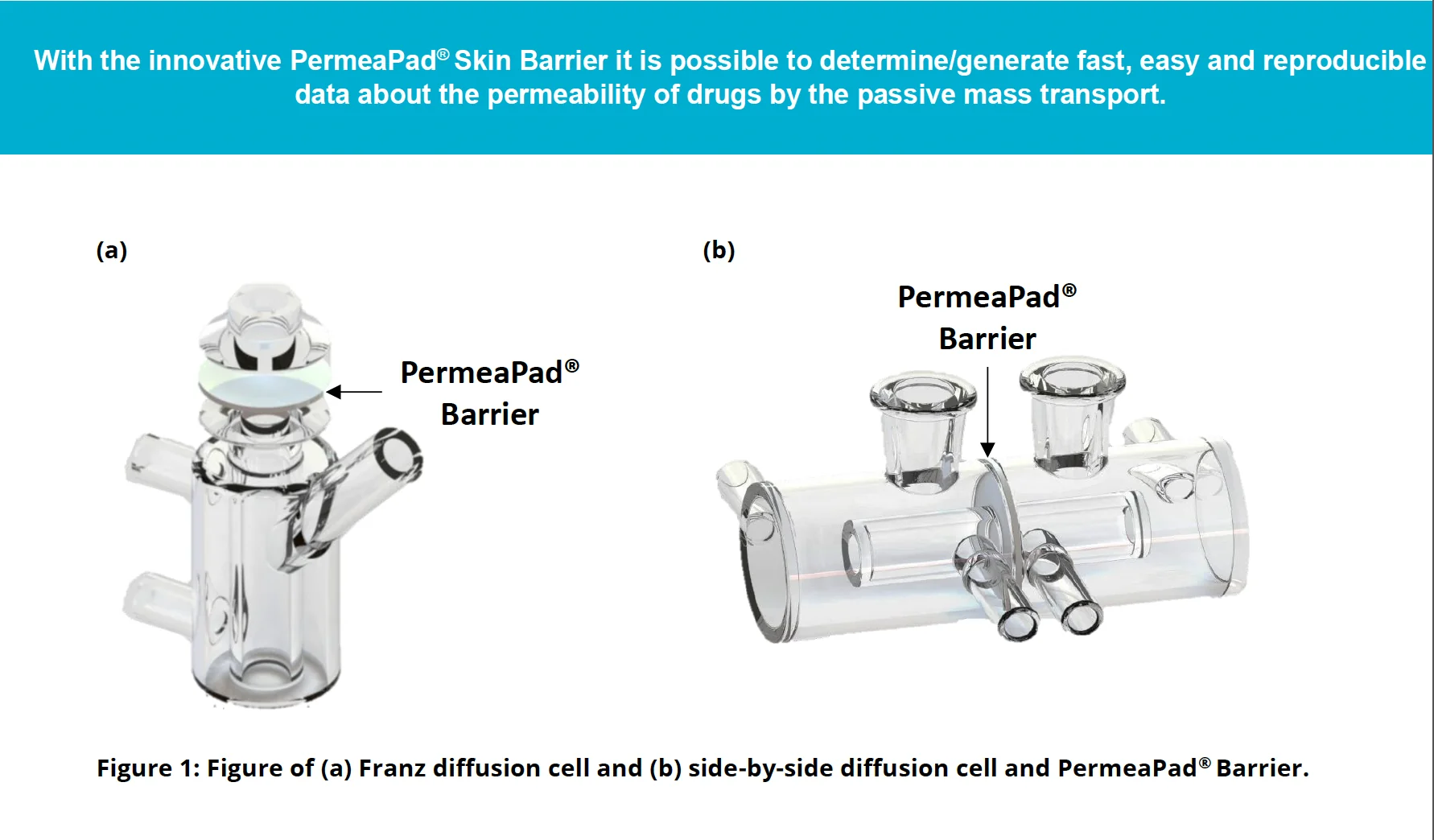
Applications
- Skin penetration studies for topical and transdermal formulations
- Dermatology research into barrier function and drug transport
- Pharmaceutical development of creams, gels, and patches
- Regulatory submission support with validated, reproducible permeability data
Scientific Validation (University of Trieste)
- Strong correlation between PermeaPad® and biological skin models for permeability of caffeine and diclofenac.
- Demonstrated as a reliable and reproducible alternative to human and animal skin.
- Batch 1.2 shows high consistency across physicochemical properties of tested drugs.
- For research use only. Not for use in diagnostic procedures.
Specifications
| Parameter | Specification |
|---|---|
| SKU | 300525 |
| Disk Diameter | 25.0 ± 0.2 mm |
| Membrane Composition | Cellulose membrane + skin-mimicking lipid mixture + cellulose filter (white) |
| MWCO | 8–10 kDa |
| Operating Temperature | 25 °C or 37 °C |
| Drug Concentration | e.g. 5 mM |
| Sampling Intervals | Freely selectable |
| Test Duration | Up to 24 hours |
| Analysis Method | HPLC, LC-MS/MS |
| Validated Drug Substances | Caffeine (gel), Diclofenac (gel), Ibuprofen (gel) |
| Storage | Store at 25 °C, avoid sun/UV exposure |
| Warranty | Three months from the delivery of the skin barriers |