Cancer remains a significant global health challenge, placing a heavy burden on healthcare systems worldwide. Low survival rates often stem from the limitations of early detection and treatment options. These limitations can make it difficult for patients to receive the timely and standardised care they need.
However, a beacon of hope shines brightly in the fight against cancer: biomarkers. These biological molecules within our bodies act as crucial clues, aiding doctors in various aspects of cancer management. Biomarkers not only hold the key to earlier detection, but also offer valuable insights into cancer’s characteristics and potential response to treatment. This newfound knowledge empowers doctors to make more informed clinical decisions, personalise treatment plans, and monitor how effectively therapies work. Recent advancements in biomarker analysis are leading to the development of dependable, cost-effective, and powerful diagnostic tools. This progress represents a significant leap forward in our collective effort to enhance early detection, expand access to advanced treatments, and ultimately, improve patient outcomes in the ongoing battle against cancer.
What are Cancer Biomarkers?
Cancer biomarkers are biological molecules produced by the body that are differentially expressed or affected during carcinogenesis compared with the normal state. The alterations can be due to several factors, including germline or somatic mutations, transcriptional changes, and post-translational modifications.
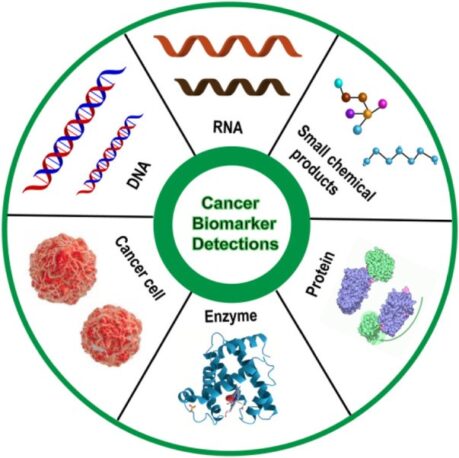
There are a variety of biomarkers, which can include proteins, DNA, RNA, and metabolites, among other categories. A biomarker can also be a collection of alterations, such as gene expression, proteomic, and metabolomic signatures.
Biomarkers can be detected in the circulation (whole blood, serum, or plasma) or excretions or secretions (stool, urine, sputum, or nipple discharge), and thus easily assessed non-invasively and serially, or can be tissue-derived, and require either biopsy or special imaging for evaluation.
Role of Cancer Biomarkers in Clinical Applications
- Screening and Early Detection: Biomarkers play a crucial role in the early detection of cancer by pinpointing specific molecules or cellular changes indicative of cancerous growth or tumours. Screening for cancer biomarkers is typically conducted in asymptomatic individuals to catch cancer at its earliest, most treatable stages, thus enhancing survival rates. Various blood-based screening biomarkers are utilised in clinical practice, such as alpha-fetoprotein (AFP) for liver cancer, Prostate-Specific Antigen (PSA) for prostate cancer, Carbohydrate antigen 19-9 (CA19-9) for pancreatic cancer, and cancer antigen 125 (CA125) for ovarian cancer, among others. However, it’s important to acknowledge that the use of some biomarkers may result in false positives and overdiagnosis. To address these limitations, multi-cancer early detection tests are being developed to complement single-cancer screening tests, offering a more comprehensive approach to cancer detection and improving diagnostic accuracy.
- Differential Diagnosis: Biomarkers serve a pivotal role in cancer diagnosis, providing crucial insights into the type, stage, and aggressiveness of the disease. They facilitate the differentiation between various cancer types and inform treatment decisions. For instance, when a patient presents with a lung nodule on chest CT, histologic evaluation of the biopsy specimen helps discern whether the tissue indicates cancer, infection, inflammation, or another benign process. However, while diagnostic biomarkers contribute significantly to cancer detection and patient classification, they are not standalone diagnostic tools. Instead, they must be combined with other diagnostic methods to establish a conclusive diagnosis.
- Prognosis: After a tumour is diagnosed, prognostic markers can provide valuable information about the likely course of the disease and patient outcomes. They help identify patients who may have a more aggressive form of cancer and require more intensive treatment, as well as those with a better prognosis who may require less aggressive treatment. For example, a high expression of the Ki-67 protein, a marker of cellular proliferation, is associated with more aggressive breast cancer and poorer prognosis.
- Prediction of Treatment Response: Biomarkers play a crucial role in forecasting how patients may respond to specific treatments like chemotherapy, targeted therapy, or immunotherapy. They enable healthcare professionals to anticipate the effectiveness of a chosen therapy before its administration, allowing for personalized treatment plans tailored to individual patients. This approach optimises treatment outcomes by maximising efficacy while mitigating potential side effects. For example, overexpression or gene amplification of the HER2 gene in breast and gastric cancers predicts for response to anti-Her2 agents such as trastuzumab, and overexpression of the estrogen receptor in breast cancer predicts for response to anti-endocrine therapies such as tamoxifen.
- Monitoring of Disease Progression: Biomarkers can be used to monitor the progression of cancer and the response to treatment over time. Changes in biomarker levels or characteristics can indicate whether the cancer is responding to treatment, progressing, or recurring.
Types of Cancer Biomarkers
- Genetic Biomarkers: Mutations or alterations in specific genes that are associated with cancer development. For example, Oncogenes are genes that can cause cancer when they are overactive or mutated. On the other hand, tumour suppressor genes are genes that normally help to prevent cancer by regulating cell growth and division and when mutated, it is deactivated and can cause carcinogenesis. HER2 amplification in breast/gastric cancers is an example of oncogene while BRCA1/2 mutations in breast/ovarian cancers are examples of tumour suppressor genes.
- Epigenetic Biomarkers: These biomarkers focus on changes that affect how genes are turned on and off, without altering the DNA code itself. DNA methylation, a prominent epigenetic modification, entails the addition of methyl groups to cytosine residues in DNA, typically occurring at CpG dinucleotides. Hypermethylation of CpG islands in gene promoter regions can result in the transcriptional silencing of tumour suppressor genes, while hypomethylation of gene bodies and repetitive DNA sequences may foster genomic instability and oncogene activation. Histone modifications represent another common epigenetic alteration where histones undergo post-translational modifications, such as acetylation, methylation, phosphorylation, and ubiquitination, thereby regulating chromatin accessibility and gene expression.
- Transcriptome biomarkers: Transcriptome cancer biomarkers are specific gene expression patterns within cells that reflect various aspects of cancer biology, such as tumor initiation, progression, metastasis, and response to therapy. These biomarkers originate from the analysis of RNA transcripts, encompassing messenger RNA (mRNA) and non-coding RNA species, including microRNAs (miRNAs), circular RNAs, and long non-coding RNAs (lncRNAs). For example, non-coding microRNA biomarkers like MiR-21 and MiR-155, as well as long non-coding RNA biomarkers such as HOTAIR and MALAT1, are frequently upregulated in diverse cancer types including breast, lung, liver, and colorectal cancer. These biomarkers serve as critical indicators of tumor progression, invasion, and metastasis.
- Protein Biomarkers: These proteins are either produced by cancer cells or elicited as a response to cancer within the body. They are valuable for both the diagnosis and monitoring of cancer. Common examples include HER2 in breast and gastric cancers, EGFR in lung, colorectal, and head and neck cancers, KRAS in colorectal, pancreatic, and lung adenocarcinoma, and PSA in prostate cancer.
- Metabolite Biomarkers: These are small molecules produced by cellular metabolism, essential for cancer cells as they undergo alterations to support their growth and survival. They serve as biomarkers for the diagnosis and monitoring of lung, pancreatic, thyroid, breast, and hepatic cancer. Metabolites encompass various classes, including nucleotides, amino acids, lipids, and carbohydrates. Examples of metabolites include palmitic acid, cholesterol, lactate, creatinine, triglycerides, urea, and ketone bodies.
- Cellular Biomarkers: Cellular biomarkers encompass specific cell types or characteristics that offer insights into the presence, progression, or response to cancer treatment. For instance, Circulating Tumor Cells (CTCs) represent cancer cells that have detached from the primary tumor and entered the bloodstream. Analyzing CTCs provides valuable information about tumor heterogeneity, metastatic potential, and treatment response. Additionally, immune cells such as Tumor-Infiltrating Lymphocytes (TILs) serve as biomarkers. The presence and composition of TILs correlate with tumor immune evasion, response to immunotherapy, and patient outcomes. Cancer Stem Cells (CSCs), a subpopulation of cancer cells with self-renewal and tumor-initiating properties, shed light on mechanisms of tumor initiation, progression, and recurrence.
- Exosomes Biomarkers: Exosomes, nano-sized particles, ferry a diverse range of functional molecules, including proteins, lipids, nucleic acids (such as DNA, messenger and noncoding RNA), and metabolites. Enhanced exosome secretion by tumor, stromal, and immune cells has been noted in cancer patients, underscoring exosomal markers as promising targets for cancer detection. For instance, elevated levels of miR-21 in circulating exosomes have been identified as potential biomarkers in various malignancies, including liver, gastric, breast, colorectal, ovarian, and esophageal cancer.
Techniques for Detecting Cancer Biomarkers
There are various tools and techniques used for detecting cancer biomarkers, each with its own advantages and limitations. Some of the common tools for detecting cancer biomarkers include:
- Immunohistochemistry (IHC): This technique involves the use of antibodies to detect specific proteins in tissue samples obtained through biopsy or surgery. IHC is commonly used to identify protein biomarkers in cancer cells and determine their presence, location, and abundance.
Check out Atlantis Bioscience Tyramide Signal Amplification
- Enzyme-Linked Immunosorbent Assay (ELISA): ELISA is a sensitive laboratory technique used to detect and quantify specific proteins or antigens in blood, serum, or other bodily fluids. ELISA kits are available for many cancer biomarkers and are widely used in clinical research and diagnosis.
Check out Atlantis Bioscience ELISA kits
- Polymerase Chain Reaction (PCR): PCR is a molecular biology technique used to amplify and detect specific DNA sequences. In cancer research, PCR can be used to identify genetic mutations, gene expression patterns, and alterations in DNA methylation associated with cancer development and progression. Various PCR-based methodologies, including real-time PCR and digital PCR, offer versatile tools for precise analysis in cancer studies.
Check out Atlantis Bioscience EvaGreen Dye
- Next-Generation Sequencing (NGS): NGS technologies enable the rapid sequencing of DNA and RNA molecules, allowing for the comprehensive analysis of genetic mutations, gene expression profiles, and epigenetic changes associated with cancer. NGS platforms are used to identify novel biomarkers, characterise tumour heterogeneity, and guide personalised treatment strategies.
Check out Atlantis Bioscience DNA and RNA quantitation kits
- Flow Cytometry: Flow cytometry is a powerful technique used in cancer biomarker detection. It identifies cell surface markers via fluorescently labelled antibodies specific to cancer-associated biomarkers, assesses cellular functions like proliferation and apoptosis, and measures DNA content to unveil aneuploidy and cell cycle abnormalities. Furthermore, it detects intracellular biomarkers such as cytokines and transcription factors, allowing for simultaneous analysis of multiple intracellular biomarkers within individual cells. Its multiplex capability enables the comprehensive assessment of cancer subtypes and tumour heterogeneity.
Check out Atlantis Bioscience Flow Cytometry Reagents
- Mass Spectrometry: Mass spectrometry is a powerful analytical technique used to identify and quantify proteins, peptides, and other biomolecules based on their mass-to-charge ratio. Mass spectrometry-based proteomics approaches can be used to discover and validate protein biomarkers in cancer cells and tissues.
Check out Atlantis Bioscience Protein Analysis Solutions
- Microarray Analysis: Microarray technology allows for the simultaneous analysis of thousands of genes or proteins on a single chip. Microarray analysis is used to identify gene expression patterns, detect genetic mutations, and discover novel biomarkers associated with cancer.
Check out Atlantis Bioscience DNA and RNA quantitation kits
- Fluorescence In Situ Hybridisation (FISH): FISH is a molecular cytogenetic technique used to detect and localise specific DNA sequences within cells or tissue samples. FISH can be used to identify chromosomal abnormalities, gene amplifications, and other genetic alterations associated with cancer.
Check out Atlantis Bioscience Reactive CF Dyes for labelling
These are just a few examples of the tools and techniques used for detecting cancer biomarkers. Each method has its strengths and limitations, and the choice of method depends on factors such as the type of biomarker, sample availability, sensitivity, specificity, and cost-effectiveness. Integrating multiple approaches often provides a more comprehensive understanding of cancer biology and facilitates the development of effective diagnostic and therapeutic strategies.
Biomarkers: A Brighter Future in Cancer Care
The fight against cancer is a constant battle, but the discovery and utilisation of cancer biomarkers offer a beacon of hope. These unique biological signals empower doctors to not only detect cancer earlier, but also understand its characteristics and predict treatment response. This newfound knowledge is revolutionizing cancer care, allowing for the development of personalised treatment plans and improved patient outcomes.
As research into cancer biomarkers continues to flourish, we can expect even more advancements in the following areas:
- Multi-biomarker panels: Combining multiple biomarkers will enhance diagnostic accuracy and provide a more comprehensive picture of the disease.
- Non-invasive detection methods: Advancements in technology will lead to simpler and less-intrusive methods for biomarker detection, such as breath tests or liquid biopsies.
- Earlier intervention: Earlier detection through biomarkers will allow for intervention at a pre-cancerous stage, potentially preventing cancer altogether.
- Treatment personalisation: Biomarkers will play a crucial role in tailoring treatments to individual patients, maximizing efficacy and minimising side effects.
The collective effort towards biomarker research is paving the way for a future where cancer is not just treatable, but preventable and ultimately, a disease of the past.
References:
Das S, Dey MK, Devireddy R, Gartia MR. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors (Basel). 2023;24(1):37. Published 2023 Dec 20. doi:10.3390/s24010037
Dong F, Yan W, Dong W, et al. DNA-enabled fluorescent-based nanosensors monitoring tumor-related RNA toward advanced cancer diagnosis: A review. Front Bioeng Biotechnol. 2022;10:1059845. Published 2022 Dec 1. doi:10.3389/fbioe.2022.1059845
Gadade, D.D., Jha, H., Kumar, C. et al. Unlocking the power of precision medicine: exploring the role of biomarkers in cancer management. Futur J Pharm Sci 10, 5 (2024). https://doi.org/10.1186/s43094-023-00573-2
Henry NL, Hayes DF. Cancer biomarkers. Mol Oncol. 2012;6(2):140-146. doi:10.1016/j.molonc.2012.01.010
Wang, X., Tian, L., Lu, J. et al. Exosomes and cancer – Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 11, 54 (2022). https://doi.org/10.1038/s41389-022-00431-5
Sarhadi VK, Armengol G. Molecular Biomarkers in Cancer. Biomolecules. 2022 Jul 23;12(8):1021. doi: 10.3390/biom12081021.