- Your cart is empty
- Continue Shopping
3 Types of Cell Surface Stains: Lipophilic Dyes, Lectins & Protein Labels Explained
3 Types of Cell Surface Stains: Lipophilic Dyes, Lectins & Protein Labels Explained
- Atlantis Bioscience
- Blog
- Reading Time: 7 minutes
You want to visualise cell boundaries for your multicolour imaging experiment. Do you use a lipophilic dye, a lectin, or a surface protein stain? Each option has strengths and pitfalls, and choosing the wrong one can waste precious samples or distort results.
Membrane staining is a valuable approach for defining cell boundaries in imaging studies, but selecting the right stain is not always straightforward. Dyes must be compatible with specific conditions such as cell viability, fixation, and downstream staining requirements. The wrong choice can result in poor signal, uneven labelling, or interference with other assays.
In this blog, we outline the main types of cell surface stains, highlight their applications, and share key considerations to help you make the right decision for your research.
Why Do We Stain the Cell Surface?
Cell surface stains are fluorescent dyes that label the plasma membrane, allowing researchers to visualize boundaries, track cells, and perform immunofluorescence. The three main types are lipophilic dyes, lectins, and protein stains, each suited for different applications in live-cell imaging or fixed-sample analysis.
The plasma membrane is more than just a barrier — it defines the shape of the cell, controls interactions with the environment, and displays molecular markers that distinguish one cell type from another. Yet under a microscope, this boundary is almost invisible without staining.
By adding a surface stain, researchers can:
- Clearly define cell boundaries in dense or complex samples.
- Differentiate between cell types based on membrane composition or markers.
- Assess cell health and morphology, as membrane integrity often reflects viability.
- Track movement and interactions of cells over time.
- Highlight specific molecules on the cell surface for diagnostics or high-content screening.
In short, cell surface staining transforms invisible outlines into actionable insights — making it a cornerstone technique for cell biology, imaging, and diagnostics.
What Is Immunofluorescence and Why Does It Matter?
Immunofluorescence is a microscopy technique that uses fluorescently labeled antibodies to detect specific proteins or markers in cells and tissues. It is widely applied in cell biology, pathology, and drug discovery for identifying cell types, studying signaling pathways, and validating biomarkers.
Cell surface stains are frequently combined with immunofluorescent labeling to provide clear cell boundaries alongside marker-specific signals. For example, lipophilic dyes or lectins can outline the plasma membrane, while antibodies highlight intracellular or surface proteins of interest. When planning such multicolour experiments, researchers must ensure spectral compatibility between stains and antibody fluorophores to avoid signal overlap.
What Is the Plasma Membrane Made Of?
The plasma membrane is a dynamic structure that separates the cell’s interior from its environment while supporting communication and transport. Its key components include:
- Phospholipid bilayer: Forms the semi-permeable foundation of the membrane.
- Proteins: Embedded within the bilayer as receptors, transporters, or structural anchors.
- Cholesterol: Modulates membrane fluidity and stability.
- Carbohydrates: Linked to proteins (glycoproteins) or lipids (glycolipids), contributing to cell recognition and signalling.
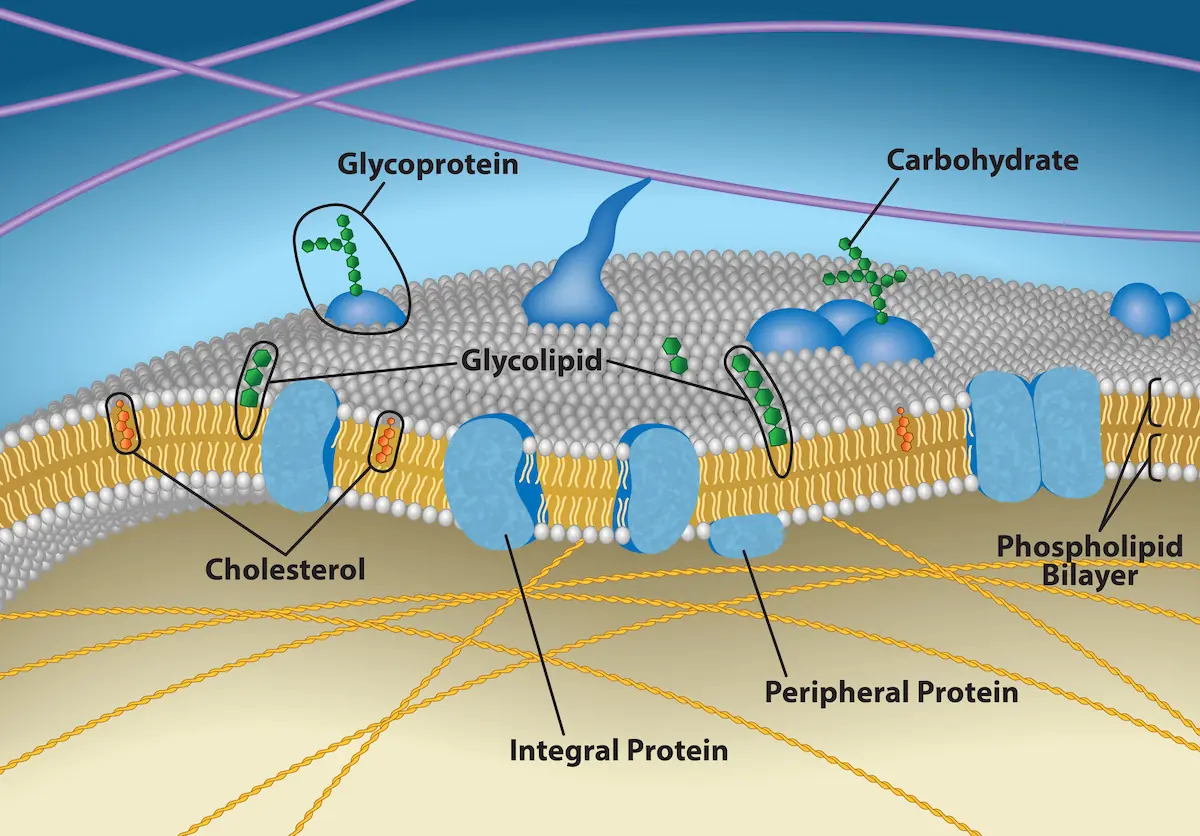
With a basic understanding of what makes up the plasma membrane, it becomes clear why different stains behave differently. Some target the lipid bilayer itself, others bind to proteins, and some recognise carbohydrate motifs on the surface. Choosing the right stain depends on your experimental goals — whether you need long-term tracking, high specificity, or compatibility with fixation and multicolour imaging.
Below, we break down the main categories of plasma membrane stains, how they work, their best applications, and key considerations for successful use.
Types of Plasma Membrane Stains and Their Applications
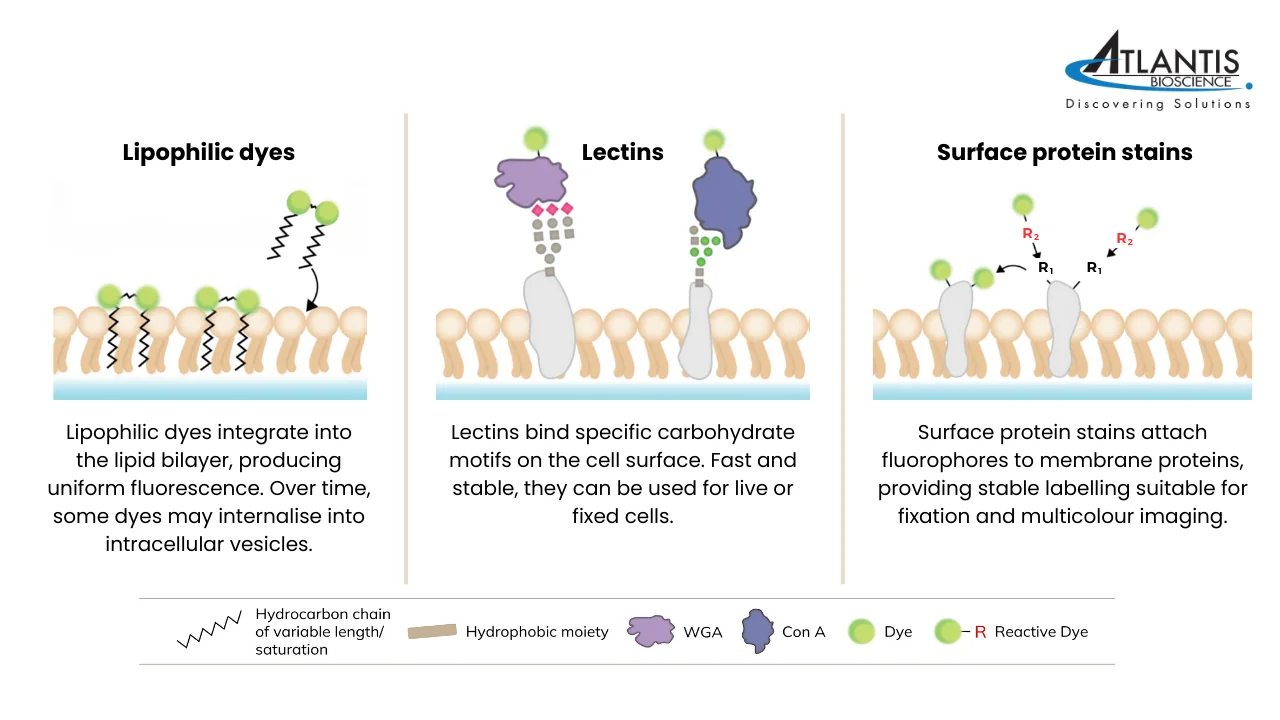
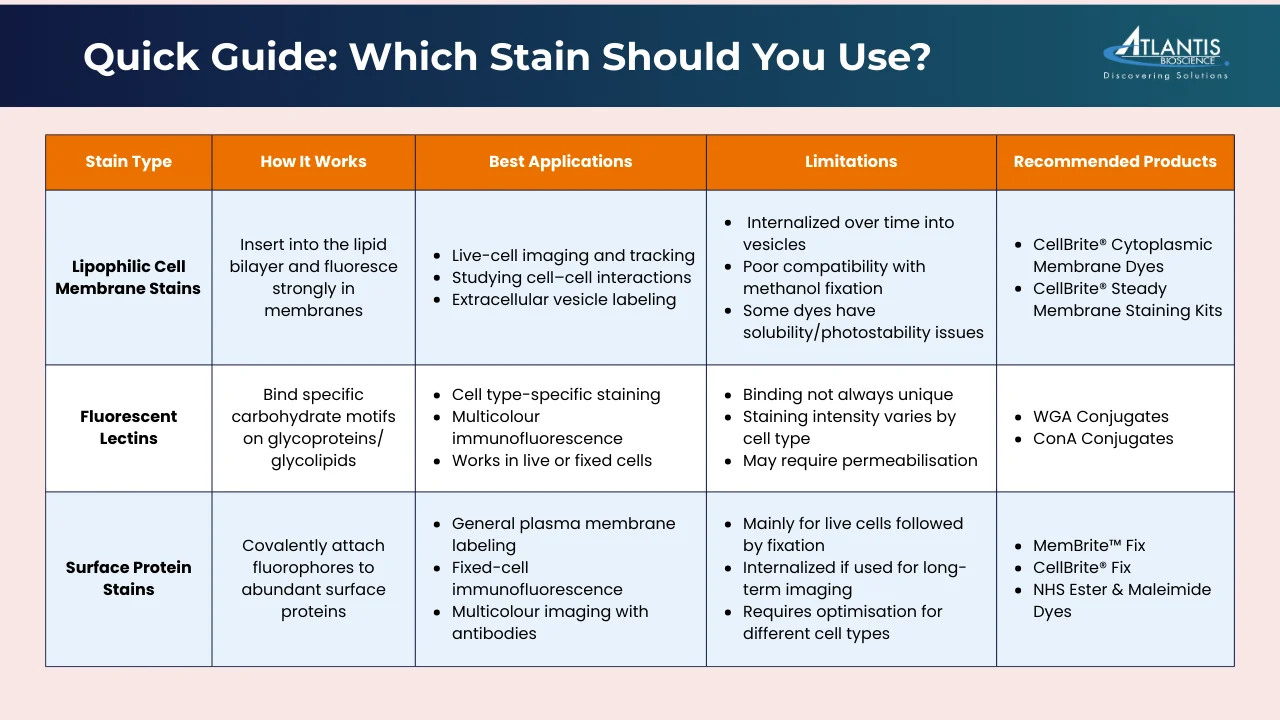
Lipophilic Fluorescent Dyes
What are they and how do they work?
Lipophilic fluorescent dyes are a class of membrane stains that integrate into lipid-rich structures, such as the plasma membrane or extracellular vesicles. Their hydrophobic tails intercalate into the lipid bilayer, while their fluorescent properties are enhanced in the lipid environment compared to aqueous solution. This results in a strong signal that enables clear visualisation of cell boundaries under a microscope. Once incorporated, these dyes diffuse laterally within the membrane, providing uniform staining at optimal concentrations. Common examples include DiO, DiI, PKH, and CellBrite® Cytoplasmic Membrane Dyes.
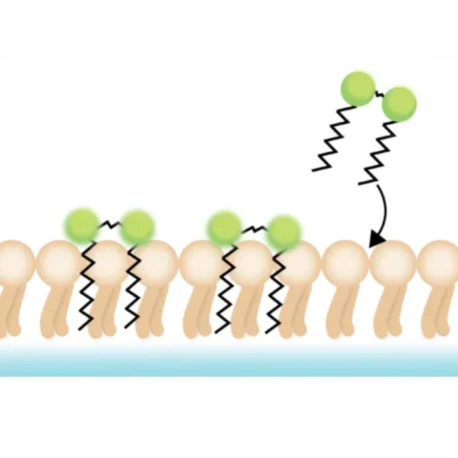
Applications
Lipophilic dyes are widely used for:
- Cell tracing and tracking – ideal for long-term monitoring of cells in culture or after transplantation.
- Cell-cell interaction studies – labelling multiple populations with different colours to study adhesion, migration, or fusion.
- Low cytotoxicity assays – their minimal impact on cell viability makes them suitable for live-cell imaging and pre-immunofluorescence workflows where cell surface integrity is essential.
Considerations
While lipophilic dyes offer stable labelling, they come with some limitations:
- Internalisation over time – although they initially label the plasma membrane, dyes are gradually internalised via endocytosis, leading to localisation in intracellular vesicles or lysosomes within hours to days.
- Fixation compatibility – these dyes work with paraformaldehyde (PFA) fixation but are poorly compatible with methanol fixation, permeabilsation, or FFPE sections due to lipid extraction during processing.
- Photostability and solubility issues – traditional carbocyanine dyes (e.g., DiD, DiR) can be difficult to dissolve, prone to aggregation, and may show inefficient or punctate staining.
CellBrite® Cytoplasmic Membrane Dyes are next-generation lipophilic stains designed to overcome many of the limitations of traditional carbocyanine dyes. The CellBrite® NIR Dyes have long 18-carbon hydrophobic tails and an additional water-soluble group, which make the dyes easy to dissolve while providing highly stable cytoplasmic membrane staining, unlike traditional carbocyanine dyes.
For experiments requiring extended plasma membrane visualisation, CellBrite® Steady Membrane Staining Kits are recommended. These formulations distribute between the cell surface and intracellular compartments, maintaining visible surface staining over time—something traditional dyes struggle to achieve.
Meanwhile, for studies where fixation and downstream immunofluorescence are essential, Cytoliner™ Fixed Cell Membrane Stain offers a solution. Unlike conventional lipophilic dyes that are highly variable due to the poor solubility of the dyes, Cytoliner dyes are a new generation of membrane dyes uniquely engineered to permit selective staining of the plasma membrane in fixed and mildly permeabilised cells.
Lectins
What are they and how do they work?
Lectins are carbohydrate-binding proteins that recognise and attach to specific sugar residues present on the cell surface. They are commonly available as conjugates with fluorophores, making them useful for direct visualisation under a microscope.
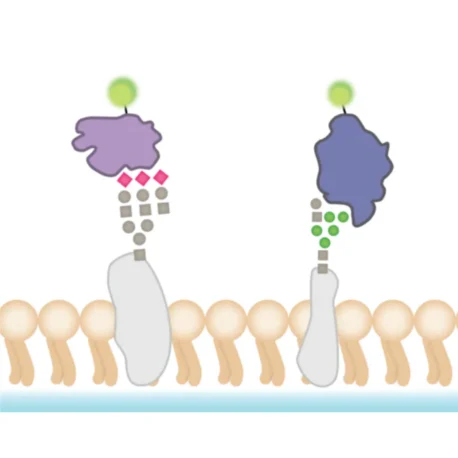
Two widely used examples are:
- Wheat Germ Agglutinin (WGA): Binds to terminal N-acetylglucosamine and sialic acid residues on proteins and sphingomyelin.
- Concanavalin A (ConA): Recognises α-D-mannosyl and α-D-glucosyl residues, which are internal components of many glycans.
Because lectins can form multiple non-covalent interactions, their overall binding strength (avidity) is high, even if each individual interaction is relatively weak. This multivalency ensures that staining is stable with low risk of dye transfer between adjacent cells. Importantly, lectin staining is fast, straightforward, and compatible with both live and fixed cells, and can also withstand permeabilisation steps.
Applications
- Cell type-specific staining: Different lectins target distinct carbohydrate motifs, which can help differentiate cell types or detect specific cell states.
- Broad utility: WGA conjugates are often used to label yeast bud scars, bacterial cell membranes, and mammalian cell surfaces.
- Multiplexing: Since multiple lectins with different fluorophores can be used in parallel, they are well suited for multicolour immunofluorescence imaging experiments.
Considerations
- Variability: Staining intensity can vary between cell types depending on the abundance and accessibility of specific carbohydrate targets.
- Intracellular use: Some applications may require permeabilisation to allow lectins to access sugars located inside the cell.
- Specificity limits: While lectins bind defined carbohydrate motifs, their recognition is not always unique to one type of cell or glycan structure.
Surface Protein Stains
What are they and how do they work?
Non-specific surface protein staining refers to the direct chemical attachment of fluorophores to endogenous proteins on the outer cell surface. Instead of using protein tags or antibodies, this approach relies on broadly reactive chemistries that modify abundant functional groups on surface proteins (for example, primary amines or thiols), producing a covalent or otherwise very stable fluorophore–protein linkage localized to the cell exterior. Unlike lipophilic dyes, which can diffuse between membranes, surface protein stains form covalent or highly stable associations that prevent dye transfer between cells. This results in more reliable and consistent labelling, particularly in mixed or co-culture systems.
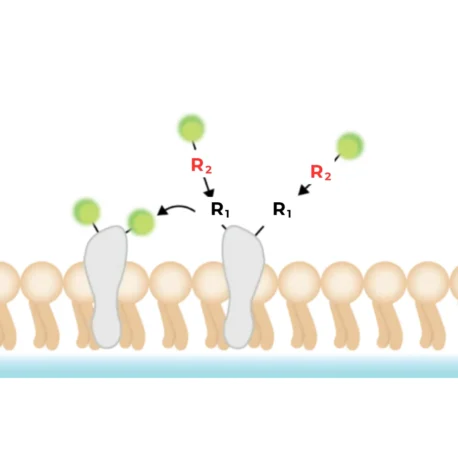
Some commercial examples are:
- MemBrite™ Fix: A two-step kit that provides bright, fixation-compatible membrane labelling in multiple colours. The exact mechanism is proprietary.
- CellBrite® Fix: Another option used for fixed-cell membrane staining (mechanistic details proprietary).
- Custom reagents: Many standard fluorophores are sold functionalised as NHS esters, maleimides, or click-compatible probes — useful when you need a specific dye or wavelength.
Applications
- General membrane labelling for imaging cell shape, boundaries, and interactions.
- Multicolour fluorescence imaging, as stained cells can be fixed and permeabilised afterwards for use with antibodies or other dyes.
Considerations
- These stains are primarily intended for live-cell use, followed by fixation; they are not suitable for directly labelling already fixed samples.
- The dyes can be internalized relatively quickly, limiting their use in long-term surface labelling experiments.
- Optimisation may be needed for certain cell types to achieve consistent staining.
Common Pitfalls to Avoid
- Using the wrong fixation: Lipophilic dyes are lost with methanol fixation or permeabilisation — stick with PFA.
- Expecting lectin specificity: Lectins bind sugar motifs, but not always uniquely — use controls to confirm.
- Overlooking dye internalisation: Both lipophilic dyes and protein stains can be endocytosed, so they are not ideal for long-term surface-only studies.
- Skipping optimisation: Different cell types vary in membrane composition; always test staining concentration and incubation time before scaling up.
- Not accounting for immunofluorescence compatibility: Some cell surface stains can mask antibody epitopes or compete for emission channels, reducing signal clarity. Always check stain chemistry and emission spectra when planning multicolour immunofluorescence labeling to avoid cross-interference.
Conclusion
No single stain works for every experiment. Lipophilic dyes are best for live-cell tracking, lectins excel for carbohydrate-rich surfaces and multicolour studies, while surface protein stains provide fixation-compatible, stable labelling. By matching your stain choice to your experimental goals — whether it’s live-cell imaging, fixed-sample analysis, or immunofluorescence protocols — you can avoid common pitfalls and generate clearer, more reliable results.
References
Hassdenteufel S, Schuldiner M. Show your true color: Mammalian cell surface staining for tracking cellular identity in multiplexing and beyond. Curr Opin Chem Biol. 2022 Feb;66:102102. doi: 10.1016/j.cbpa.2021.102102.
Xu S, Pan W, Song ZL, Yuan L. Molecular Engineering of Near-Infrared Fluorescent Probes for Cell Membrane Imaging. Molecules. 2023 Feb 16;28(4):1906. doi: 10.3390/molecules28041906.
CONTACT

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotation.
OTHER BLOGS YOU MIGHT LIKE
HOW CAN WE HELP YOU? Our specialists are to help you find the best product for your application. We will be happy to help you find the right product for the job.

TALK TO A SPECIALIST
Contact our Customer Care, Sales & Scientific Assistance

EMAIL US
Consult and asked questions about our products & services

DOCUMENTATION
Documentation of Technical & Safety Data Sheet, Guides and more...
