- Your cart is empty
- Continue Shopping
EMBER500™ RNA Prestain Loading Dye
EMBER500™ RNA Prestain Loading Dye
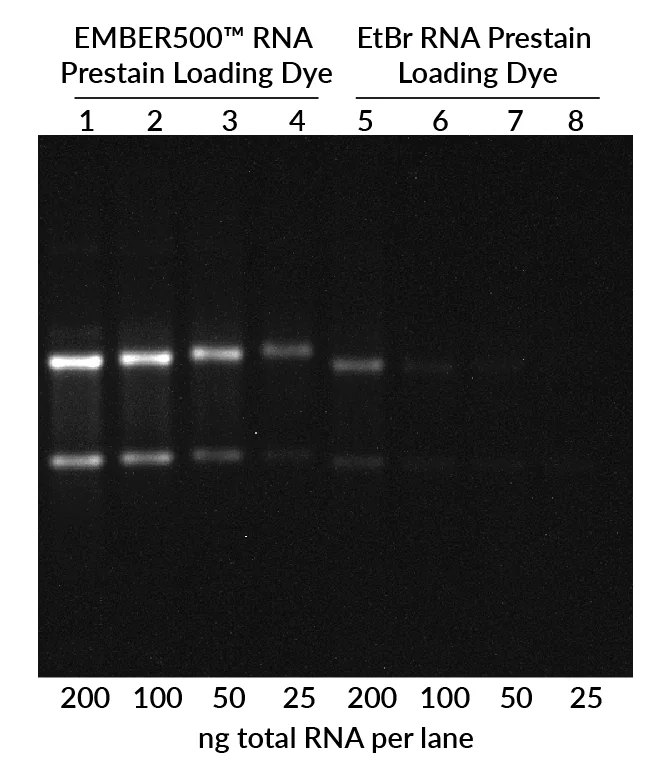
EMBER500™ RNA Prestain Loading Dye provides single-step denaturing, staining, and loading of RNA, delivering brighter sensitivity than ethidium bromide on standard agarose gels.
Bright, Convenient RNA Prestain Loading Dye for Gel Electrophoresis
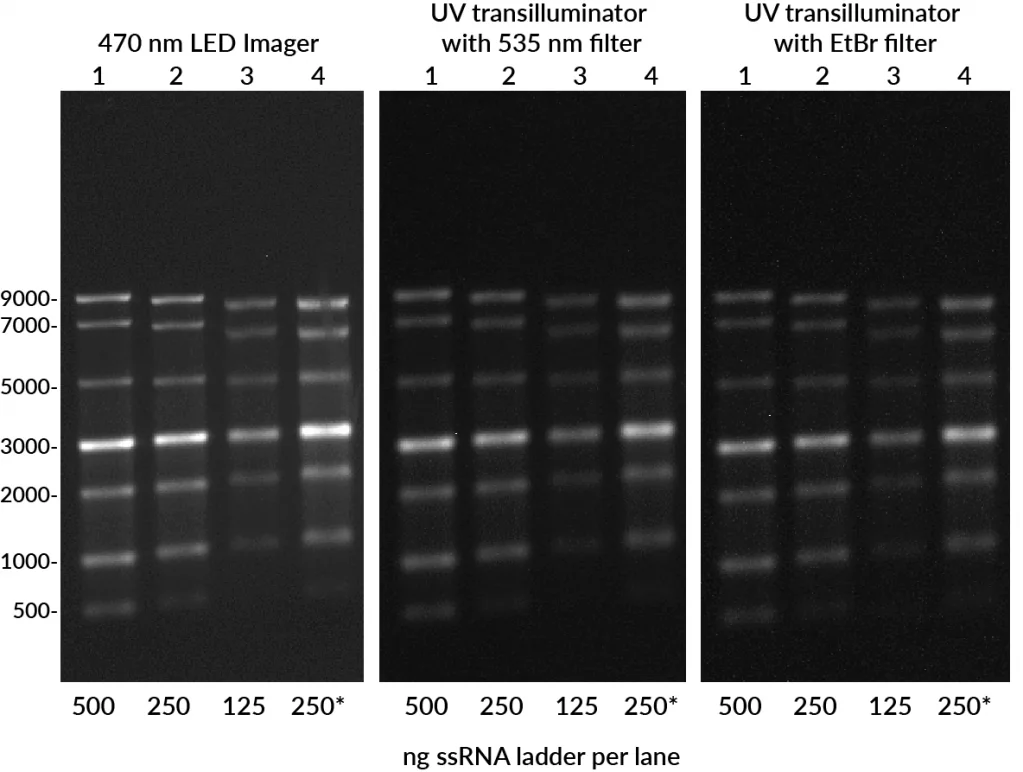
Evaluating RNA integrity is critical for gene expression and transcriptomics workflows, yet traditional staining methods like ethidium bromide (EtBr) offer limited sensitivity. EMBER500™ RNA Prestain Loading Dye streamlines RNA analysis with a single-step solution that denatures, stains, and loads RNA directly onto a regular agarose gel.
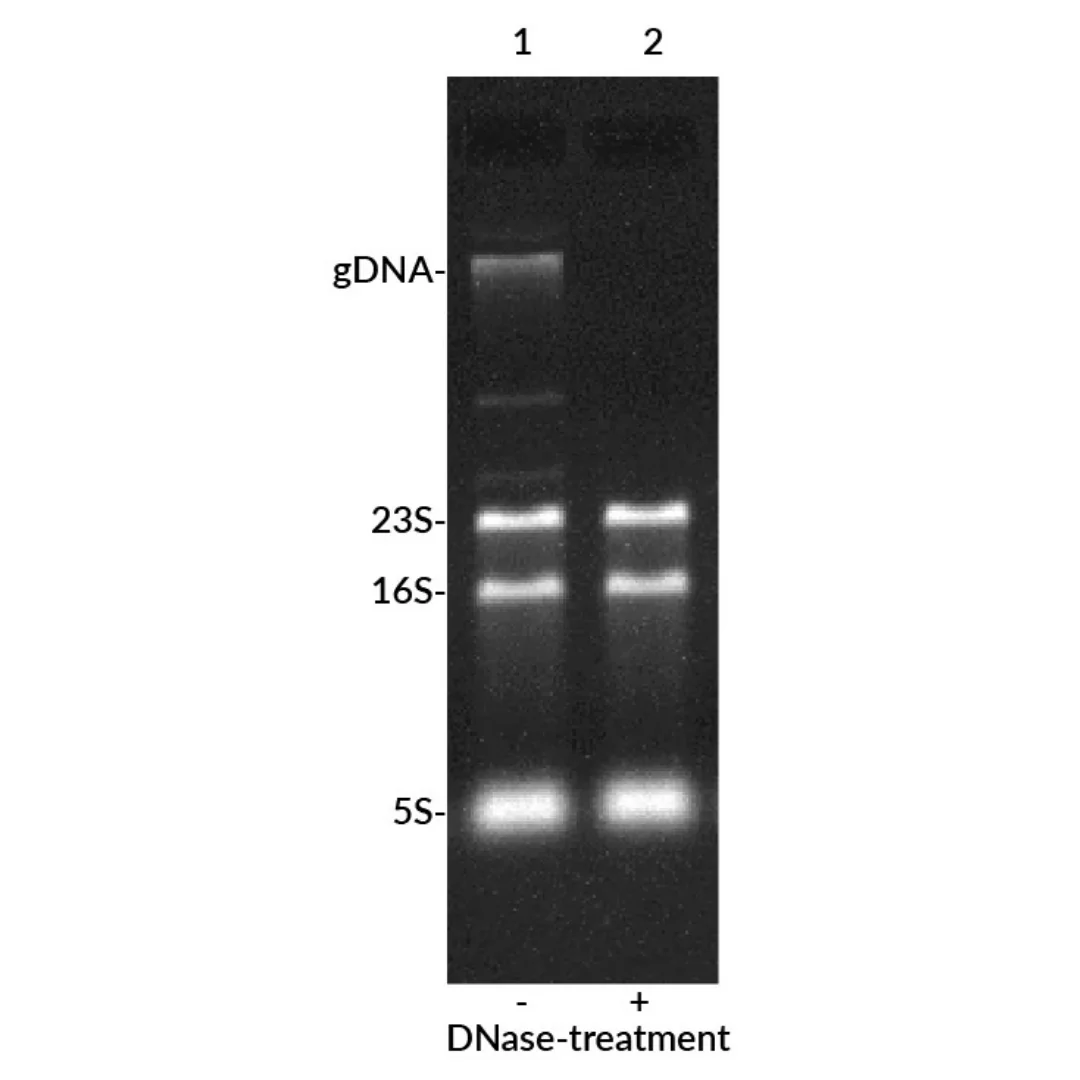
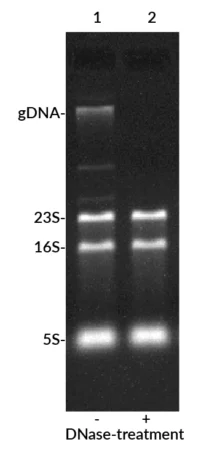
The result: faster, simpler, and far more sensitive detection compared to EtBr prestaining, without the need for complex and hazardous denaturing gels. EMBER500™ also stains DNA, allowing researchers to quickly identify genomic DNA contamination in RNA samples.
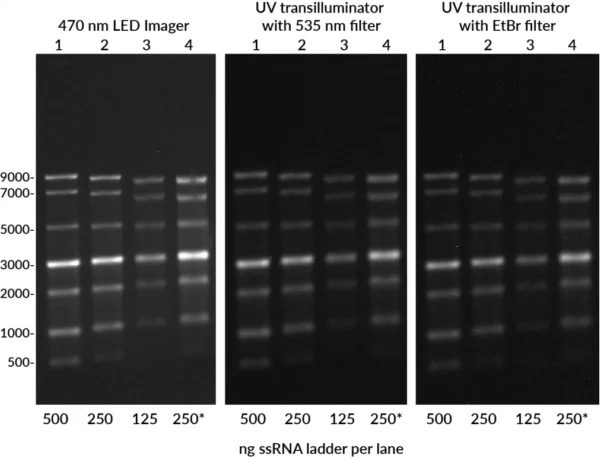
Compatible with both UV and blue LED gel imagers, EMBER500™ offers broad flexibility while avoiding UV safety hazards.
Key Features
- Fast & convenient: Single-step denaturing, staining, and loading of RNA.
- Superior sensitivity: Brighter, more sensitive than EtBr prestaining.
- RNA integrity check: Enables detection of RNA quality and genomic DNA contamination.
- Instrument compatibility: Works with UV or blue LED imagers and standard filters.
- Safer workflow: Eliminates the need for hazardous denaturing gels.
- Tracking dyes included: Bromophenol blue (~300 bp) and xylene cyanol (~3 kb) for electrophoresis monitoring.
Applications
- Evaluation of total RNA integrity
- Detection of DNA contamination in RNA preps
- Gel electrophoresis of RNA for downstream workflows (qPCR, RNA-seq, IVT)
- General RNA quality control in molecular biology labs
Notes
- Not recommended for analysing low molecular weight RNA or ssDNA.
- Not compatible with formaldehyde, glyoxal, or urea denaturing gels.
- For maximum sensitivity (detecting as little as ≤5 ng RNA), use the EMBER™ Ultra RNA Gel Kit.
Format sizes:
- 4 × 1 mL (Cat. #41032)
- 250 µL (Cat. #41032-250uL)
✅ For research use only. Not for use in diagnostic procedures.