- Your cart is empty
- Continue Shopping
Preclinical Efficacy Services GemPharmatech
Our dedication to advancing preclinical research goes beyond providing innovative mouse models; we are also committed to supporting our customers by offering preclinical efficacy services. Our experienced scientists determine the safety and efficacy of your compounds using our models and facilities, ensuring that testing is controlled and that you receive reliable and quality data. GemPharmatech is currently offering efficacy services for oncology-immunology, metabolism, autoimmune diseases, and many others.
PRECLINICAL TESTING SERVICES
Bioanalytical Services
Our in vitro platform provides comprehensive testing services, including both a flow cytometry platform and a cell analysis platform. The flow cytometry platform is equipped with a Thermo Attune NxT flow cytometer (up to 14 channels) and a GentleMACS tissue processor. GemPharmatech offers in vivo services such as mouse immune cell typing, tumor-infiltrating lymphocytes (TILs) analysis, immune reconstitution in humanized mice, as well as cell-based testing services, including antibody binding assays, anti-cancer drug target screening, and cell proliferation assays.
Service specifications
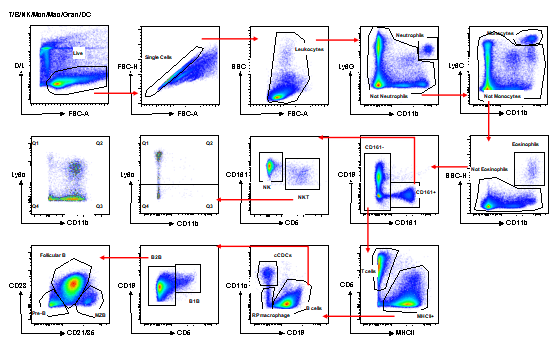
1. Standard murine FACs panel
Murine Spleen Immune Cell Gating Strategy
2. TILs Analysis
By analyzing the infiltration of immune cells from tumours, Tumor-Infiltrating lymphocytes (TILs) analysis assesses the pathways by which drugs exert their anti-tumor effects.
TILs Analysis of Syngeneic Mouse Models
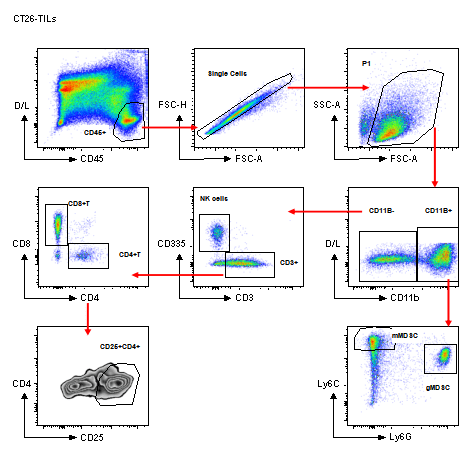
Standard Murine TIL Panel
Murine TIL Gating Strategy
3. Antibody binding assay
Used to evaluate the binding efficiency of antibody-based drugs with targets corresponding to humanized cell lines and humanized mice, this method compares the effects between the test article and the positive control, providing a reference for in vivo effects of the test article.
3.1 Cetuximab binding with A431 cells
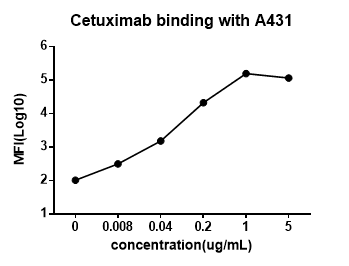
Following the increasing concentration of Cetuximab at a low level, the amount of the drug bound to the cells increased, resulting in higher fluorescent signal detection. Further increase of the drug concentration after reaching the saturation concentration did not enhance the fluorescence signal detection.
3.2 Tecentriq binding with MC38-hPDL1 cells
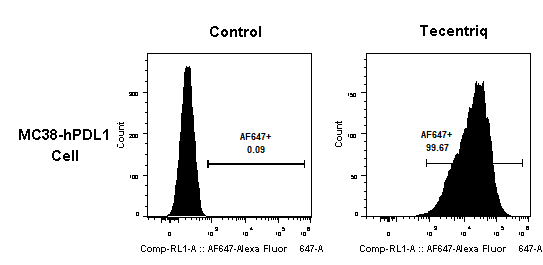
Tecentriq is a hPDL1-targeting antibody drug. After incubation of Tecentriq with MC38-hPDL1 (a cell line modified by GemPharmatech), Anti-human IgG fluorescent secondary antibody was added to detect the binding efficiency between the drug and the cells by flow cytometry.
Phenotyping Services
In 2012, GemPharmatech joined the International Mouse Phenotyping Consortium (IMPC), and in 2013, we completed the creation of an international phenotyping platform that meets international standards.
The standard analysis process begins at 9.5 days of embryonic development for phenotypic screening of mice. It continues up to 16 weeks, encompassing twenty major tests of cardiovascular, five senses, energy metabolism, bone, neurobehavior, blood biochemistry, blood cells, histomorphology, and others. Based on the phenotype analysis platform and self-developed novel models (such as severe immunodeficient NCG mice and immune checkpoint humanized mice), we have developed a phenotype analysis service system that includes tumor, metabolism, and overall phenotype screening and pharmacological pathology.
Oncology Platform
GemPharmatech has successfully developed multiple PDX models, which cover cancers such as gastrointestinal, liver, pancreatic, lung, and hematologic, for a wide variety of uses, including efficacy evaluations, clinical studies as treatment control, and pathogenesis studies. While conducting efficacy evaluations of immunomodulators like hPD-1/hPD-L1 and hCTLA4 antibodies, the company utilized mouse models with humanized immune systems prepared from PBMCs and HSCs by PDX and CDX techniques. Mouse models with humanized immune checkpoints require no reconstruction of the immune system; therefore, using such models in the preclinical evaluation of the immunomodulators is both more economical and more convenient.
They are capable of monitoring tumor volume and weight and providing a basis for research in hematology, clinical biochemistry, immunohistochemistry, and others.
Metabolism Platform
GemPharmatech provides animal disease models and genetically humanized models for metabolic diseases, including diabetes mellitus, abnormal lipid metabolism, obesity, and NASH. The company additionally provides a series of in vivo R&D services, with corresponding disease models selected in light of the mechanism of action of the investigational drug.
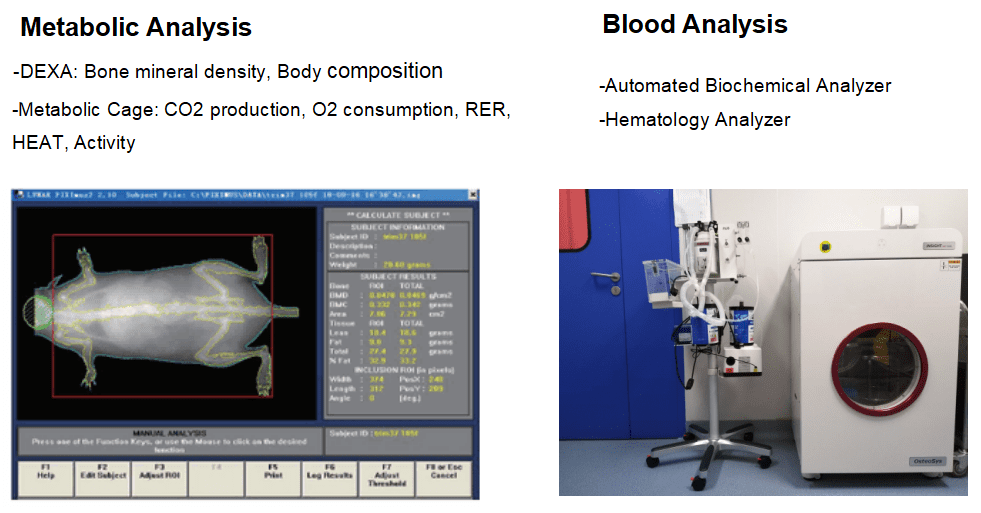
These models include histopathological analysis, detection of metabolism-related cytokines and inflammatory factors, body weight and food intake, blood glucose, glycosylated haemoglobin (HbA1c), plasma insulin, GTT, and ITT, as well as body composition, bone density, and both indirect and direct metabolic cage tests, which monitor for factors such as CO2 and O2 production, heat, RER and activity, or urine and fecal collection.
Efficacy and Pathology Platform
To promote bioscience research and accelerate the biopharmaceutical R&D process, GemPharmatech is committed to histopathology assays in animal models for a variety of diseases, gene knockout and knock-in mouse models, and other animal studies. Our platforms offer services in multiple pathology fields, including histology, molecular pathology, and clinical pathology. We offer a one-stop shop for sample collection, fixing, embedding, section preparation, staining and special staining, image interpretation, and pathology reports.
|
Immunochemistry staining procedures |
Staining procedures of special structures |
|
| Immunohistochemical staining, IHC | Safranin O | Sirius Red |
| Immunofluorescence staining, IF | Oil Red O | Prussian Blue |
| Immunocytic staining, ICC | Giemsa | Orcein Elastin |
| IHC double staining | Bielschowsky | Congo Red |
| IF double staining | Alcian Blue | Alizarin Red |
| TdT-mediated dUTP Nick End Labeling (TUNEL) | PAS | Methenamine Silver |
| Toluidine Blue | Luxol Fast Blue | |
| Nissl | Microglia | |
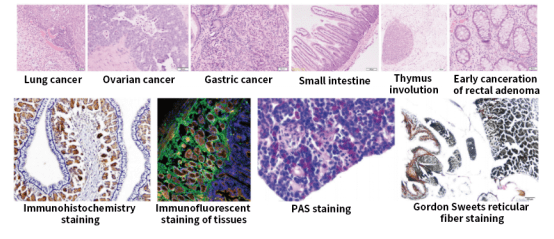
Anti-Tumor Drug Efficacy Evaluation
GemPharmatech’s comprehensive in vivo efficacy evaluation platform for antitumor drugs facilitates the development of immuno-oncology therapies. Our team can perform a variety of tests, including 14 marker flow cytometry, TIL analysis, small molecule testing, bioassays, and non-GLP toxicology studies.
Humanized Immune Checkpoint Mouse Models and Efficacy Services
Immune Checkpoint Inhibitors represent a prominent hot spot in the R&D of anti-tumor drugs, where the efficacy and safety evaluations of immune checkpoint inhibitors require ideal animal models. Though a perfect animal model is expected to have an intact immune system, as immune checkpoint therapies rely on the immune system to achieve their actions, immune checkpoints have different antibody recognition sites due to their varied molecular conservatism across animal species. Therefore, drug efficacy and safety evaluation in animal models with humanized immune checkpoint modules yield a higher clinical conversation rate.
By establishing a series of mouse models with humanized immune-checkpoint genes and independent intellectual property rights, which are developed by replacing the extracellular regions of murine immune checkpoint molecules with the corresponding human sequences, GemPharmatech models are capable of broad recognition and evaluation of antibodies targeting human immune checkpoints while simultaneously preserving transmembrane and intracellular regions of the murine immune checkpoint genes to ensure the intact and accurate intracellular signaling. These humanized mice are ideal models for efficacy and safety evaluation of immune checkpoint inhibitors.
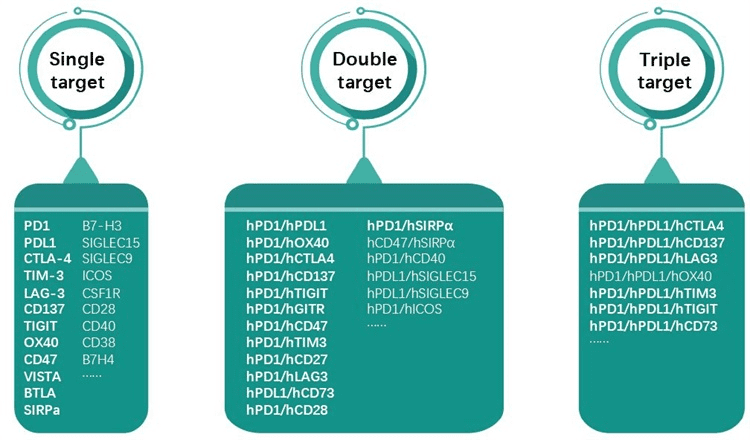
GemPharmatech offers the following 30+ humanized mice with dual genetic backgrounds (BALB/c and C57BL/6):
Non-GLP Toxicity Assessment Services
Because BALB/c mice are more susceptible to immune-related adverse events (irAEs), GemPharmatech provides immune-checkpoint inhibitor-related toxicity evaluations. The irAEs of immune-checkpoint inhibitors can be evaluated by clinical observation of mice, pathological evaluation, testing of cytokines, and monitoring of clinical indexes dynamically during the dosing process, all of which provides an effective toxicity evaluation platform for preclinical research of ICIs.
BALB/c-hCTLA4 Models
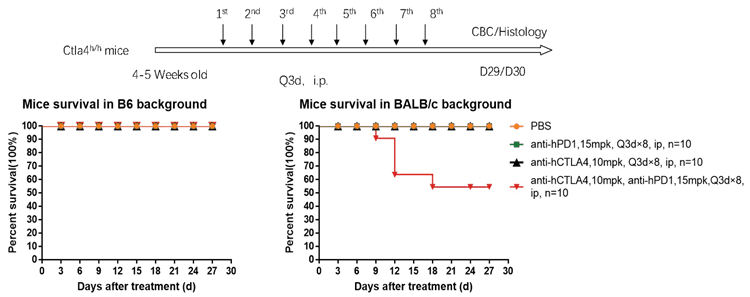
The toxic side effects of anti-PD1 + anti-CTLA4 combination therapy were evaluated with C57BL/6-hPD1/hCTLA4 and BALB/c-hPD1/hCTLA4 mice, respectively.
1. The survival curve of the PD1 antibody + Yervoy combo therapy in B6 and BALB/c mice showed that BALB/c mice died on Day 9 and were not tolerant until Day 18 in the study.
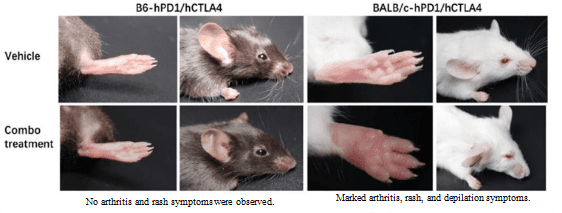
2. Clinical trial data for the CTLA4-targeting drug Yervoy showed that patients suffered severe side effects, including dermatitis, hepatitis, diarrhea, and rectocolitis. BALB/c mice exhibited pathological symptoms such as arthritis, rashes, and depilation, but B6 mice exhibited no such symptoms.
3. During the clinical biochemical assessment of Yervoy, the levels of lactate dehydrogenase (LDH) and creatine kinase (CK) were significantly higher in BALB/c mice than in B6 mice, indicating that the cardiac damage produced by Yervoy was more severe in BALB/c animals.
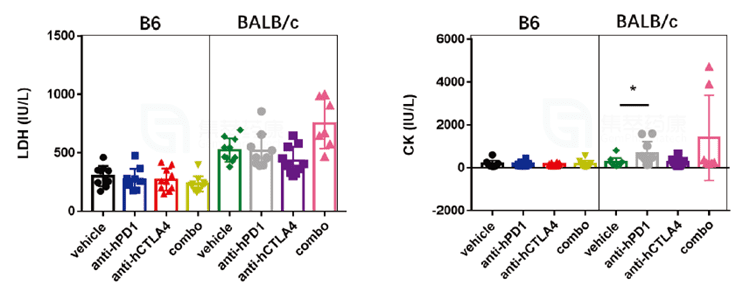
4. Yervoy caused inflammatory injury on mice hearts to varied severity. Inflammatory lesions observed in BALB/c murine hearts were more severe than in B6 background mice.
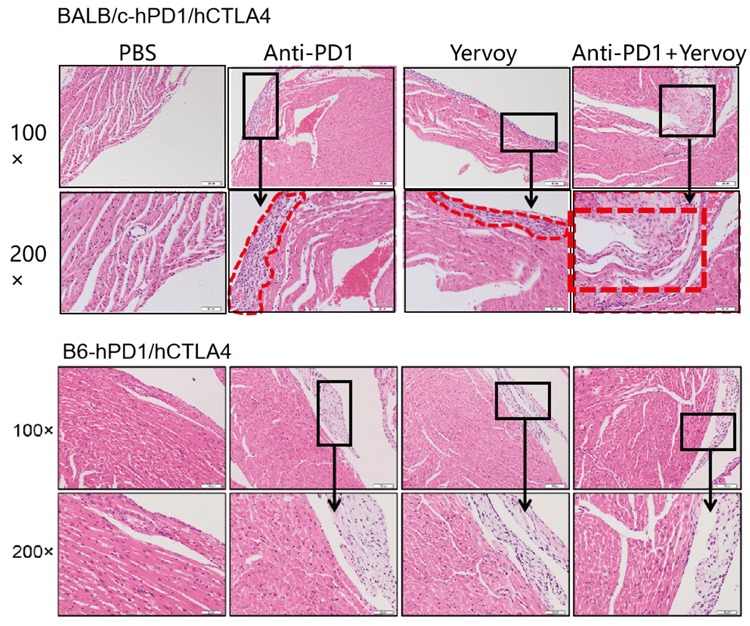
B6-hPD1/CTLA4 mouse models proved tolerant to drug therapies; humanized immune checkpoint BALB/c-hPD1/hPD1 mice make ideal models for evaluating macromolecular and small-molecule drug toxicity in preclinical trials due to their ability to reproduce certain adverse reactions (ARs) in clinical practice.
Metabolic Disease Efficacy Evaluation
GemPharmatech performs a variety of metabolic efficacy tests, including glycated hemoglobin assays, glucose and insulin tolerance tests, metabolic cage tests, and blood biochemistry analyses. We have developed a series of mice strains with several metabolic diseases, including obesity, diabetes, non-alcoholic steatohepatitis (NASH), and hyperlipidemia, as well as simulated human diseases by medication induction and feeding manipulation. Based on these models, we conduct research on the pathophysiology of associated disorders, drug screening, and efficacy evaluation.
Obesity Models and Efficacy Services
Obesity is a kind of metabolic disorder affected by many factors such as diet, environment and heredity. Besides weight gain, it may also lead to a series of complications such as diabetes, hypertension, hyperlipidemia and heart disease. In order to further study the obesity mechanism and evaluate related therapeutic drugs, GemPharmatech has developed a series of obesity mouse models, including diet-induced obesity model and spontaneous obesity model caused by gene mutation, and can provide drug efficacy evaluation services based on these models.
Obesity Models
- 60% HFD diet-induced obesity (DIO) model: 60% high-fat diet-induced modeling.
- 45% HFHSD diet-induced obesity (DIO) model: 45% high-fat, high-sugar diet-induced modeling.
- Gene mutation obesity mouse model C57BL/6-Alms1-del: Alms1 gene fragment deletion, resulting in a combined phenotype of obesity, diabetes, and non-alcoholic fatty liver disease.
- Wild mouse 750: Conventional diet feeding can cause spontaneous obesity; 60% high-fat diet feeding can shorten the modeling cycle..
- Obesity-related target humanized mice.
Type II Diabetes Models and Efficacy Services
Diabetes mellitus is a common disease caused by genetic, environmental, and immunological factors resulting in islet hypofunction and insulin resistance, and revealing complex metabolic disorders involving sugar, protein, fat, water, and electrolytes. More than 90% of patients with diabetes have type II diabetes(T2D). GemPharmatech has generated the matching diabetes mellitus models using gene-editing technology and a diet-induced modeling approach for researching type II diabetes mellitus pathogenesis as well as evaluating therapeutic medication screening.
Type II Diabetes Models
- Single gene mutant mouse model BKS-db: Four-week-old leptin receptor (Ldlr)-deficient BKS-db mice display indications of type II diabetes, including hyperglycemia and hyperinsulinemia.
- HFHSD+STZ induction model: Combining a high-fat-high-sugar diet with a low-dose injection of streptozotocin (STZ) induces T2D symptoms in C57BL/6J mice, which can be used to evaluate the efficacy of anti-T2D medicines.
- Humanized mouse model of type II diabetes-related targets.
Liver Disease Models and Efficacy Services
The liver is an essential hub for material and energy metabolic processes. The impact of alcohol, drugs, and toxic chemicals usually produces acute or chronic liver injury, which can progress to liver fibrosis, cirrhosis, or liver cancer if not appropriately managed. Consequently, experimental animal models of liver disease are essential for investigating the pathophysiology of liver disease and screening therapeutic medicines.GemPharmatech offers liver fibrosis models, non-alcoholic steatohepatitis models, and related-efficacy evaluation services.
Liver Fibrosis Models
Liver fibrosis refers to a pathological process marked by the excessive deposition of extracellular matrix in the liver and serves as an intermediate in the progression of several liver disorders. Selecting appropriate animal models of liver fibrosis is imperative to understanding the etiology of liver fibrosis as well as selecting the most efficient screening method for anti-liver fibrosis medicines. GemPharmatech established drug-induced liver fibrosis models, which can be utilized to examine the therapeutic effects of medications on liver fibrosis.
- CCl4-induced liver fibrosis model
Atherosclerosis Models and Efficacy Services
Atherosclerosis is a chronic disease caused by the deposition of fat, cholesterol, calcium, and other chemicals in the endothelium of arteries, resulting in the loss of arterial elasticity due to vessel thickening and sclerosis. Once thick plaque substantially blocks the arteries, it can significantly limit blood flow to the vessels serving the tissues, resulting in severe tissue damage. GemPharmatech has developed ApoE knockout models and Ldlr knockout models that can be used to study atherosclerosis and efficacy evaluation services for the related drugs.
Atherosclerosis Models
- ApoE KO model: ApoE KO mice were constructed by knocking out ApoE in C57BL/6JGpt mice using CRISPR/Cas9 technology and induced by a Western diet to promote the development and progression of atherosclerosis.
- Ldlr KO model: Ldlr KO mice were constructed by knocking out Ldlr C57BL/6JGpt using CRISPR/Cas9 technology and induced by a Western diet promoted the development and progression of atherosclerosis.
- Atherosclerosis-related target humanized mouse models.
Test items
- Body weight, blood lipids, pathological tests (immunohistochemistry, special staining, etc.),
- Protein immunization, qRT-PCR, etc.
Hyperlipidemia Models and Efficacy Services
Due to improvements in standard of living and the evolution of average dietary structures, the occurrence of hyperlipidemia (HLP) is increasing. HLP poses a grave threat to human health because it can lead to the development of metabolic disorders in the body, which in turn cause cardiovascular diseases such as atherosclerosis, coronary heart disease, and myocardial infarction. The prevention of hyperlipidemia and the development of related therapeutic medications are of incredible significance.
Hyperlipidemia Models
- B6-hPCSK9 mouse model: A humanized model generated by replacing the PCSK9 coding region of C57BL/6JGpt mice with the human counterpart. These mice can develop hyperlipidemia after feeding a high-cholesterol diet and are useful for evaluating PCSK9-targeting antibody drugs.
- B6-hPCSK9-UTR mouse model: The coding region of the PCSK9 gene and the 3′ UTR region of C57BL/6JGpt mice are humanized by full fragment substitution to construct the B6-hPCSK9-UTR mouse model, which can develop hyperlipidemia after feeding a high-cholesterol diet and is suitable for the evaluation of siRNA drugs targeting PCSK9.
Gout/Hyperuricemia Models and Efficacy Services
Hyperuricemia is a common purine metabolic disorder caused by excessive synthesis or decreased excretion of uric acid as a result of abnormalities in the purine metabolic pathway. A significant risk factor for the development of gout, Hyperuricemia also increases the risk of cardiovascular disease and chronic renal failure. GemPharmatech has developed gout and hyperuricemia models via gene editing and drug induction, and can also provide model-based therapeutic efficacy evaluation services. By injecting uric acid into the local tissues or joints of mice, we have additionally successfully reproduced the symptoms of acute gout in humans.
- Uox KO gout mouse model
- MSU-induced gouty arthritis mouse model
Test Items
- Observation of the degree of mice paw swelling
- Detection of inflammatory factors in blood (ELISA Kit), histopathological analysis (H&E staining, etc.), gene expression analysis (qRT-PCR & WB).
Autoimmune Disease Efficacy Evaluation
GemPharmatech’s preclinical service platform offers our clients one-stop technical services for preclinical autoimmune disease drug development. Our experienced scientific team, rich mouse model resources, and stringent quality control standards provide a comprehensive system for preclinical services, including preclinical efficacy evaluation and customized services to support IND submission.
Neurodegenerative Disease Efficacy Evaluation
Neurodegenerative diseases, including Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), and Huntington’s disease (HD), are irreversible disorders caused by the loss of neuronal structure or function. Our development of FAD4T (AD), SNCA (PD), TDP43 (ALS), SOD1 (ALS) and HTT (HD) mice, created as independent intellectual property, provides a behavioral analysis platform for the conduction of numerous tests, including the Morrise water maze, Y maze, and gait analysis. GemPharmatech additionally offers efficacy evaluation services for several neurodegenerative diseases to expedite the discovery of therapeutic drugs for these conditions.
In Vitro Testing
GemPharmatech offers histological analysis and molecular analysis.
| Histological Analysis | Molecular Biological Analysis |
|
|
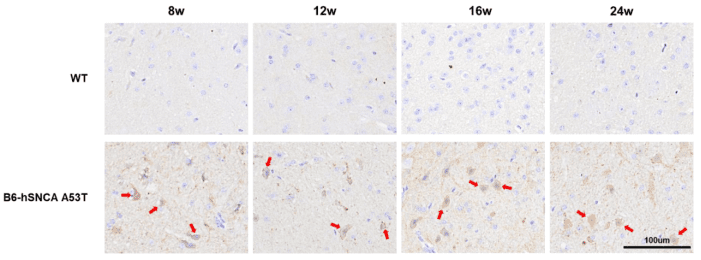
Pathologic phenotypes of B6-hSNCA A53T mice with IHC detection. Cytoplasmic aggregation of a-synuclein protein in midbrain region can be detected in B6-hSNCA A53T mice at 8 weeks of age.
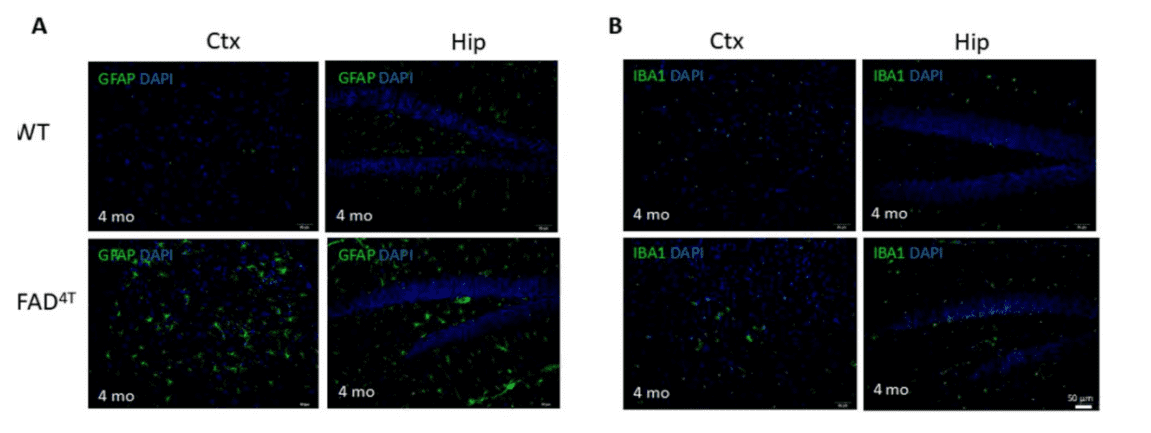
Proliferation of astrocytes and microglia were observed in the hippocampus and cortex of FAD4T mice at 4 months of age.
In Vivo Biochemical Examining
GemPharmatech’s in vivo biochemical examination platform provides metabolic analysis, blood analysis, and other testing services.
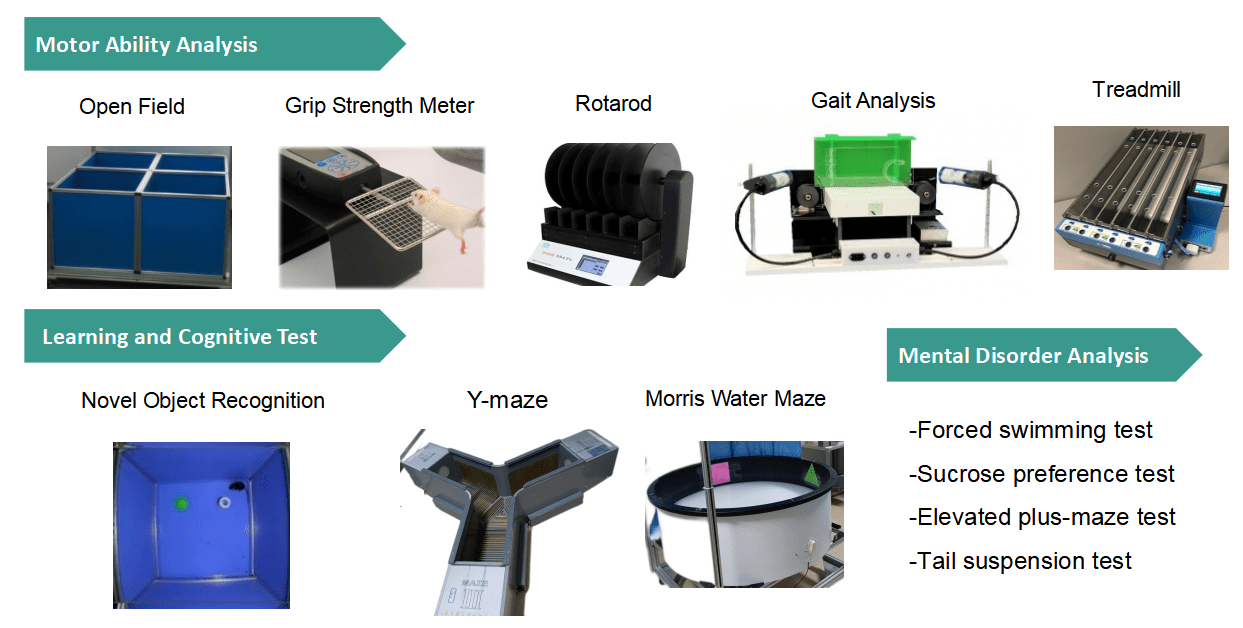
Behavioral Analysis
Available Services
- Learning and memory ability testing: open field test (OFT), object recognition test, Y-maze test, and the Morris water maze, etc.
- Locomotor ability testing: grip strength, rotarod, treadmill, pole climbing, etc.
- Depression testing: sucrose preference test, OFT, tail suspension test, forced swimming test, etc.
- Anxiety testing: OFT, elevated-plus maze test, etc.
- Behavior analysis program: TopScan LITE (CleverSys, the only four-point tracking system in the industry). This method tracks nose, body core, center of body weight, tail simultaneously, which provides research data on range, position, movement distance, and speed for a more in-depth analysis.
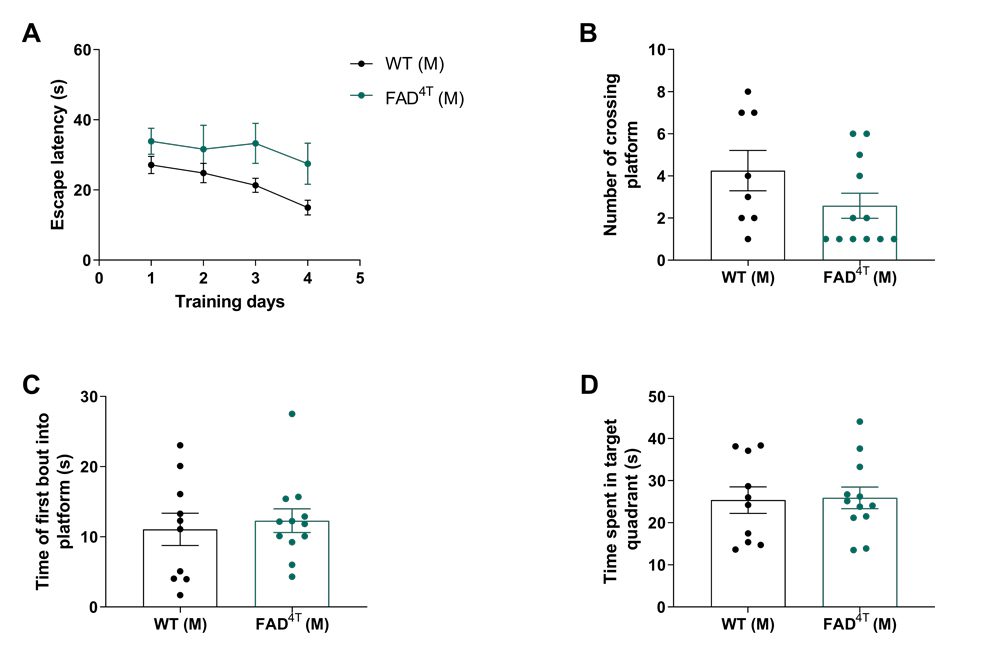
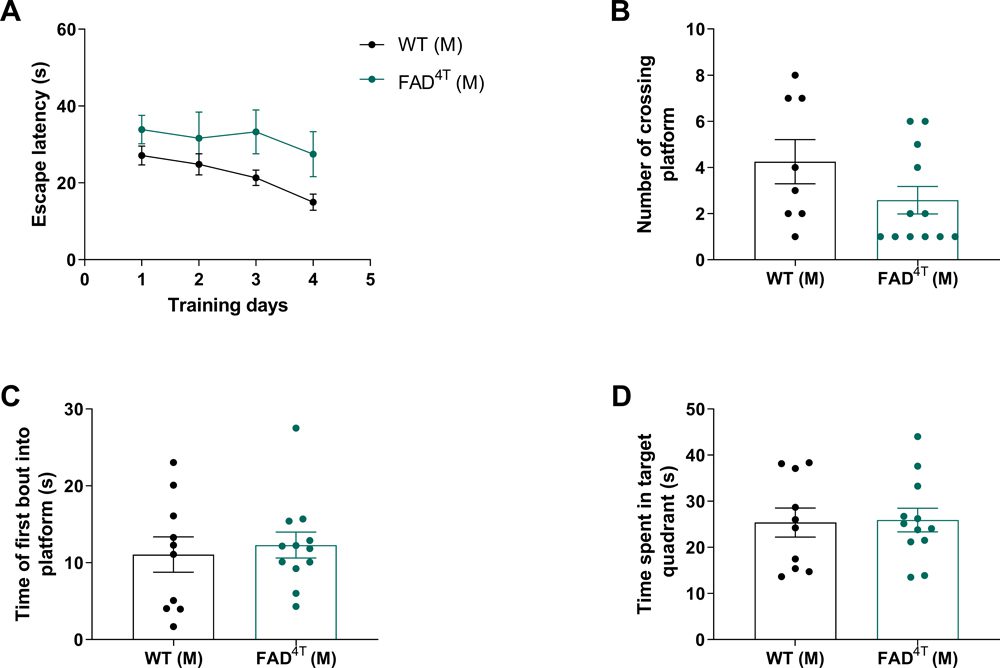
Detection of learning and memory ability of FAD4T mice. Validation of spatial learning and memory impairment in FAD4T female mice at 8-9 months of age.
In Vivo Drug Efficacy Evaluation
GemPharmatech conduct functional testing of the nervous system and behavioral analyses utilizing mouse models of neurological diseases.
Cell Therapy & Gene Therapy Efficacy Evaluation
The rapid growth of biotechnology drove the maturation of both cell therapy and gene therapy. Cell therapy, categorized according to the type of cells used for treatment, includes both stem cell therapies and immune cell therapy, such as CAR-T, TCR-T, and CAR-NK. The most significant of these varieties is CAR-T therapy.
Chimeric Antigen Receptor T cell therapy, known as CAR-T cell therapy, is a novel technique that utilizes adoptive immunotherapy to treat tumors. CAR is composed of three structural components: an extracellular antigen-binding domain, a transmembrane domain, and an intracellular signaling domain. Compared to conventional immunization therapies, CAR-T cells have both superior specificity and longer-lasting efficacy. These cells can recognize and target tumor antigens, demonstrating remarkable success in treating malignant tumors, particularly hematomas, and can destroy tumor cells without antigen presentation.
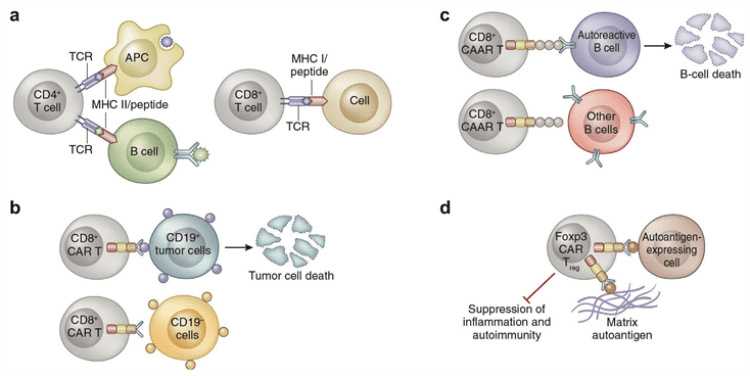
Application of CAR-T cell therapy in immune-related diseases[1].
Advantages:
The NCG mouse, a severely immunodeficient strain produced and established independently by GemPharmatech, is obtained by utilizing gene editing technologies to knock out the Prkdc and Il2rg genes of the NOD/ShiltJGpt mice. This mouse is fully devoid of T-cells, B-cells, and NK cells, and is ideal for evaluating the efficacy and safety of CAR-T therapy and related cell therapies due to its status as the most immunodeficient mouse model.
GemPharmatech has established CDX and PDX libraries with independent property rights, including a range of solid tumors and hematological system tumors, and offering an abundance of services using these tumor model resources for CAR-T therapy effectiveness research. We additionally possess a mouse in vivo imaging system, which is utilized to detect various mouse tumor models and metastatic tumor models in situ. Similarly, GemPharmatech has also developed techniques for absolute CAR-T cell counting and cytokine detection in peripheral blood, which addresses CAR-T investigations’ testing requirements.

Applications of in vivo imaging system: observation of tumor burden after in situ xenograft of hematomas and solid tumors (encephalic glioma, hepatic carcinoma, etc.), determination of tumor growth, and efficacy evaluation for test antitumor drugs.
Service Specifications:
1. In vivo efficacy test on CDX (Nalm6-Luciferase) models
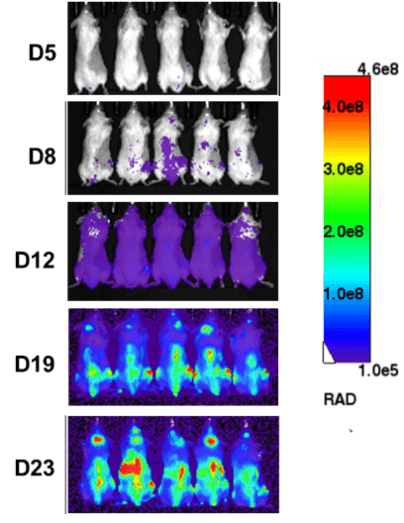
After tail vein inoculation of NCG mice with Nalm6-Luciferase cells, tumor load was detected using in vivo imaging on day 5, 8, 12, 19, and 23. The progression of tumor load in mice can be determined based on the signal intensity of in vivo imaging.
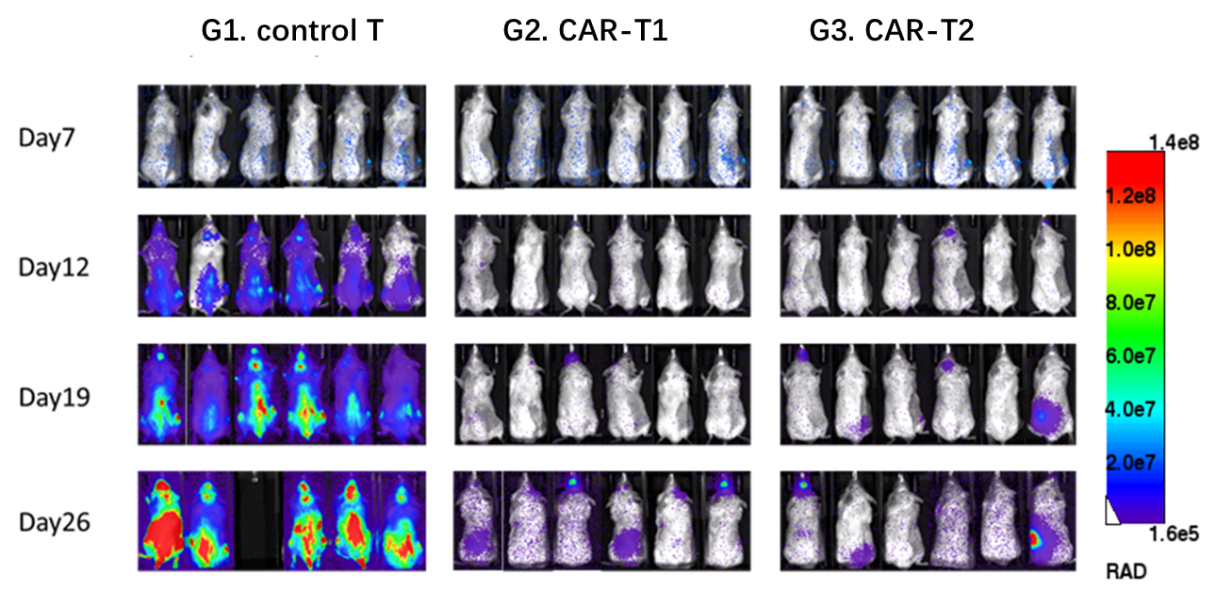
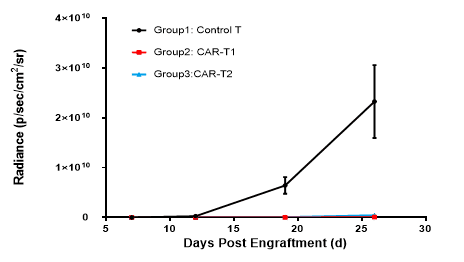
The NCG mice were inoculated intravenously with Nalm6-Luciferase cells. After 7 days, the mice were randomly allocated into three groups. Then the mice were treated with control T, CAR-T1 and CAR-T2.
CAR-T1 and CAR-T2 had obvious inhibitory effects on tumor growth (TGI, CAR-T1=100.00%, TGI, CAR-T2=100.00%), indicating NCG mice as the ideal animal model to evaluate the efficacy of CAR-T.
References
[1] Chimeric antigen receptor T (CAR T) cells: another cancer therapy with potential applications in kidney disease and transplantation? Kidney Int., 2018, 94(1): 4-6.
For more information, please contact us [email protected]
Want To Inquire About The Services?
Contact Us

THE ATLANTIS BIOSCIENCE DIFFERENCE Discover Translational Solutions To Advance From Bench to Bed
GET SUPPORT Whenever You Need It

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotation.