- Your cart is empty
- Continue Shopping
Express Gene-to-AAV Production Service in Southeast Asia
Tired of long turnaround times and unreliable AAV production?
Our Express Gene-to-AAV service offers a streamlined and efficient solution to accelerate your research. By combining expert design, rapid production, and rigorous quality control, we deliver high-quality AAV vectors in just 4 weeks.
Our Express Gene-to-AAV Service Includes:
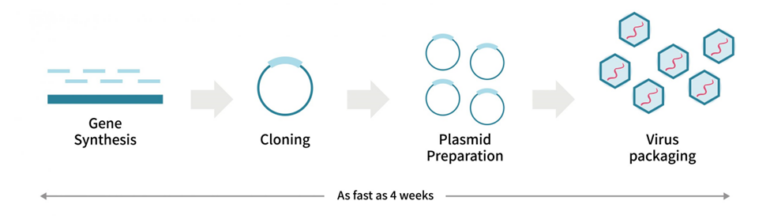
| Serotype | 60+ serotypes | Vector type | Over-expression, CRISPR, shRNA, miRNA, etc. |
| AAV type | ssAAV, scAAV | Promoter | CMV, CAG, EFS, hSyn, U6, H1 etc. or custom promoter |
| Packaging plasmid | Enhanced yield with PackGene-provided RC and helper plasmid | Gene of Interest | Custom Gene, or pre-synthesized gene elements (Reporter, Gene editing, etc.) |
- Gene Synthesis and Cloning:
- Expert Design: Our experienced team designs optimal gene constructs.
- Efficient Cloning: We utilize advanced cloning techniques to ensure rapid and accurate gene insertion.
- AAV Vector Production:
- High-Titer AAVs: We produce high-titer AAVs with a range of serotypes to maximize transduction efficiency.
- Rigorous Quality Control: Our stringent quality control measures ensure purity, potency, and safety.
- Comprehensive Quality Control:
- Purity and Potency: We perform comprehensive tests to assess the purity and titer of your AAV vectors.
- Safety Testing: We conduct rigorous safety testing to ensure compliance with regulatory standards.
What do you get from our Express Gene-to-AAV Service?
- AAV Vector: High-quality AAV vector, ready for your research experiments.
- Detailed Quality Control Report: Comprehensive documentation of vector quality and performance.
- Plasmid Map and Sequence: Detailed information about the plasmid construct used for AAV production.
- Customizable Reporting: Tailored reports to meet your specific needs.
Why Choose PackGene?
- Rapid Turnaround Time: Experience accelerated timelines with our efficient production process.
- Customizable Solutions: Tailor your AAV vectors to your specific research needs, including serotype, promoter, and transgene selection.
- Unmatched Quality: Benefit from our commitment to quality, ensuring consistent and reliable results.
- Expert Technical Support: Experienced team provides guidance and support throughout the entire process.
- Competitive Pricing: Cost-effective solutions help you maximize your budget.
Case Study
Case 1
Case 2
Case 3
Case 4


- Spinal muscular atrophy with myoclonic epilepsy (SMA-PME, ASAH1 gene defect)
- Single dose, intrathecal and intracerebroventricular injection.
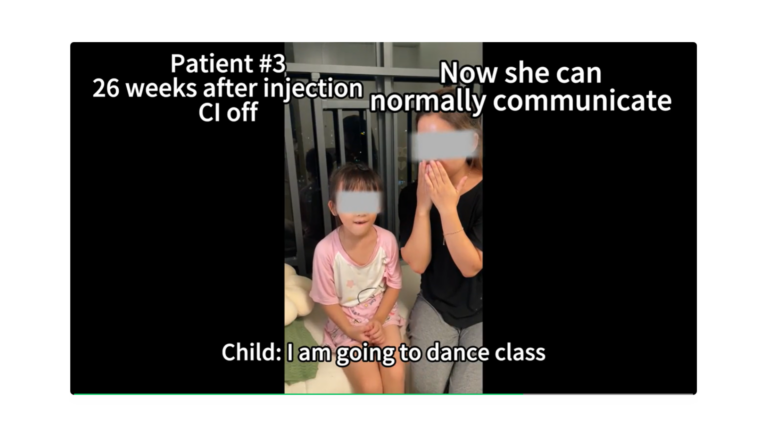

- OTOF biallelic mutations cause severe hearing impairment or complete hearing loss.
- Single dose, cochlear injection.

- Advanced blindness caused by retinitis pigmentosa.
- Single dose, intraocular intravitreal injection.

- Amyotrophic lateral sclerosis (ALS).
- Single dose, intrathecal injection.
Your Success, Our Priority
By choosing PackGene, you’re investing in a partnership dedicated to your research success. Our team is committed to providing exceptional service and delivering high-quality AAV vectors.
Contact us today to discuss your specific research needs and learn how our Express Gene-to-AAV service can accelerate your scientific discoveries.
Want To Inquire About The Services?
Contact Us

THE ATLANTIS BIOSCIENCE DIFFERENCE Discover Translational Solutions To Advance From Bench to Bed
GET SUPPORT Whenever You Need It

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotation.





