- Your cart is empty
- Continue Shopping
Fast turnaround
4-5 weeks from design to delivery
Custom Design
Tailored to your research needs
GMP-compliant production
Supporting pre-clinical and clinical development
Accelerate your drug delivery, vaccine development, and gene therapy research with Atlantis Bioscience’s custom mRNA production service, powered by PackGene.
In partnership with PackGene, a leading CRO/CDMO with over a decade of expertise in mRNA and viral vector manufacturing, we deliver both high-quality RUO and GMP-grade mRNA production tailored to the evolving needs of scientists and biotech innovators across Southeast Asia (Singapore, Thailand & Malaysia).
Workflow and Sequence
Our streamlined mRNA/LNP Synthesis Process ensures reproducibility from DNA template to drug-ready formulation:
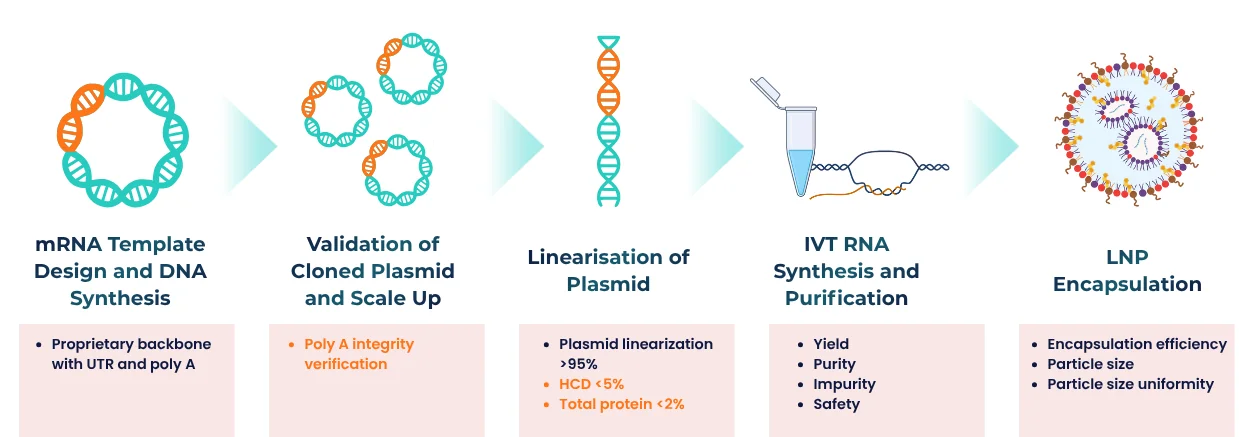
Custom mRNA production using in vitro transcription (IVT) is a proven method for synthesizing high-quality RUO mRNA and GMP mRNA from plasmid DNA templates. PackGene’s IVT process incorporates a promoter sequence, the gene of interest, and a poly(A) tail, streamlining mRNA manufacturing while ensuring consistency, scalability, and cost-effectiveness for research, preclinical, and clinical applications.
Why Choose Our Custom mRNA Services?
mRNA (messenger RNA) is revolutionizing therapeutic development as a cornerstone for vaccines, cancer treatments, and genetic disorder therapies. With its ability to enable localized expression, rapid development cycles, and unparalleled safety as a non-genome-editing platform, mRNA is driving innovation in modern medicine.
At PackGene, we provide:
- Custom mRNA manufacturing for applications like EGFP mRNA, mCherry mRNA, and other research-grade molecules.
- mRNA quality is ensured with CE testing, along with guaranteed LNP uniformity and a comprehensive QC panel.
- Seamless transition from RUO-grade mRNA production to GMP-grade mRNA manufacturing, ensuring clinical readiness.
- Industry-leading timelines and exceptional quality backed by rigorous quality control protocols.
Leverage PackGene’s expertise to streamline your mRNA production process, from early research to scalable GMP manufacturing. Let us help you accelerate breakthroughs in gene therapy and achieve impactful results.
What We are Offer
| Catalog | Capping and modification | Gene | Quantity | Timeline (Business days) |
|---|---|---|---|---|
| mRNA | Custom mRNA Cap1 | Custom gene | 100μg-20mg | 10-15 |
| mRNA | Custom mRNA Cap1 N1meΨU | Custom gene | 100μg-20mg | 10-15 |
| mRNA-LNP | Custom mRNA Cap1 in LNP | Custom gene or off-the-shelf | 100μg-20mg | 10-25 |
| mRNA-LNP | Custom mRNA Cap1 N1meΨU in LNP | Custom gene or off-the-shelf | 100μg-20mg | 10-25 |
*Additional time (~2-3 weeks ) for custom gene synthesis
mRNA Grade and QC Standard
| QC Category | QC Item | Method | Specification | Research Grade | Add-on |
|---|---|---|---|---|---|
| Identification | Appearance | Visual Inspection | Clear and free of foreign particles | √ | |
| mRNA Concentration | UV Absorbance by Nanodrop | ≥1mg/ml | √ | ||
| RNA Integrity/size | Capillary Electrophoresis | Target ±30%, Expected band size detected | √ | ||
| Buffer Specification | Client Spec | RnaseFree H2O(default), PBS, 1mM Sodium Citrate, pH 6.4 | √ | ||
| Purity | A 260/280 Ratio | UV Absorbance by Nanodrop | 1.70-2.30 | √ | |
| Size based purity | Capillary Electrophoresis | >80% | √ | ||
| Impurity | Total Protein residue | Nano Orange | ≤1% | √ | |
| Plamsid DNA residue | qPCR | ≤0.1% | √ | ||
| dsRNA | Slot-blot | ≤1% | √ | ||
| Safety | Endotoxin | Semiquantitative LAL | <10EU/mg | √ | |
| Endotoxin | Quantitative | <10EU/mg | √ | ||
| Bioburden | Direct Inoculation | No growth after 48 hrs | √ | ||
| Raw material-Linearization | Linearization percentage | AGE | >95% | √ | HPLC |
| Host cell DNA | Quantitative PCR | ≤ 5% | √ | ||
| Total protein | Nano Orange | ≤1% | √ | ||
| RNA | AGE | Non-detectable by gel electrophoresis at 200ng | √ | ||
| Raw material -circular Plasmid | Gene of Interest | Sanger sequencing | 100% math reference sequence (not including poly A) | √ | |
| Poly A | Sanger sequencing | ≤110A ±5nt, ≤111-125A ±8nt | √ | ||
| Poly A Length | Enzyme digestion and CE | Target ±5% | √ |
Performance
RNA Integrity
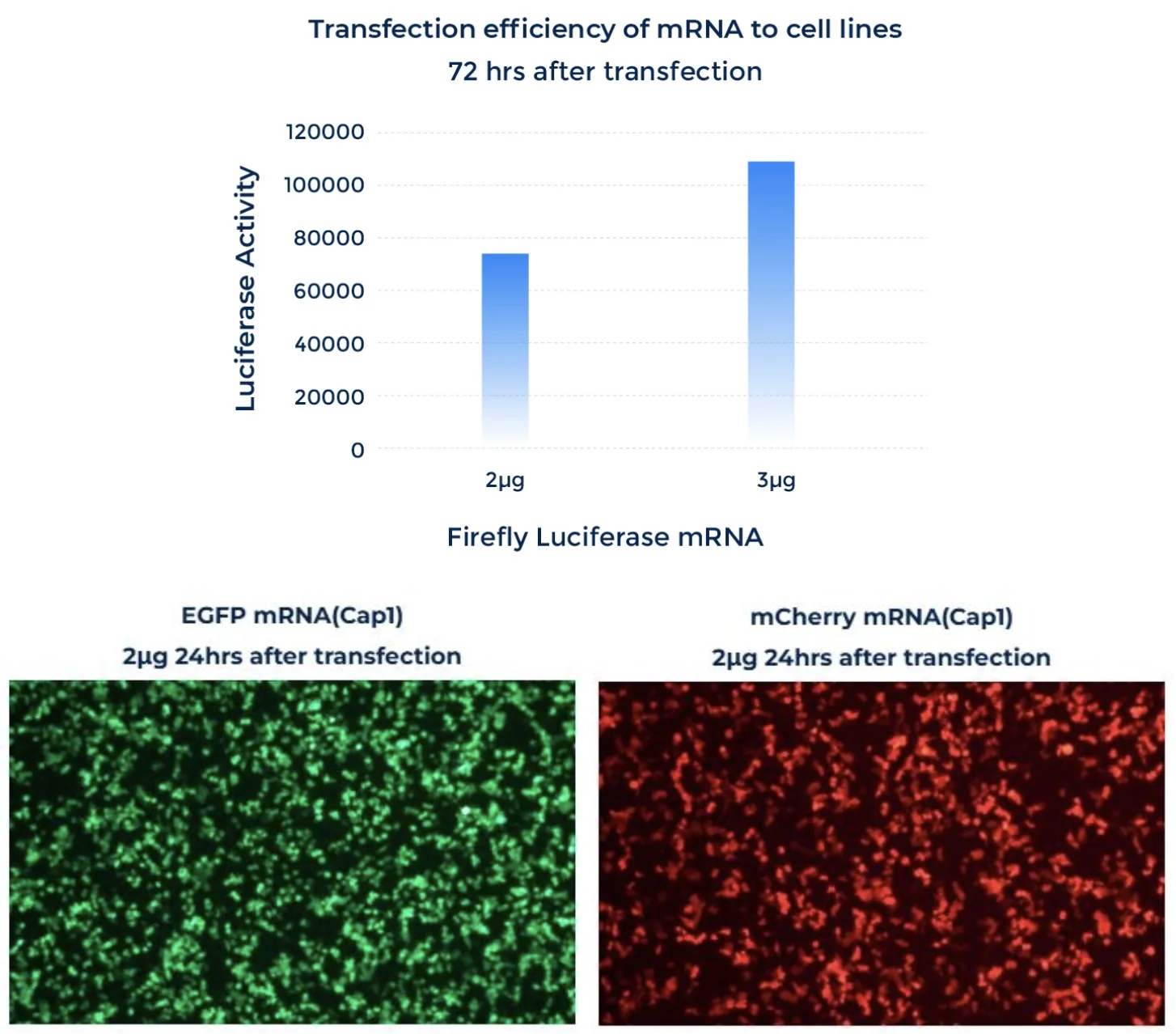
mRNA Expression
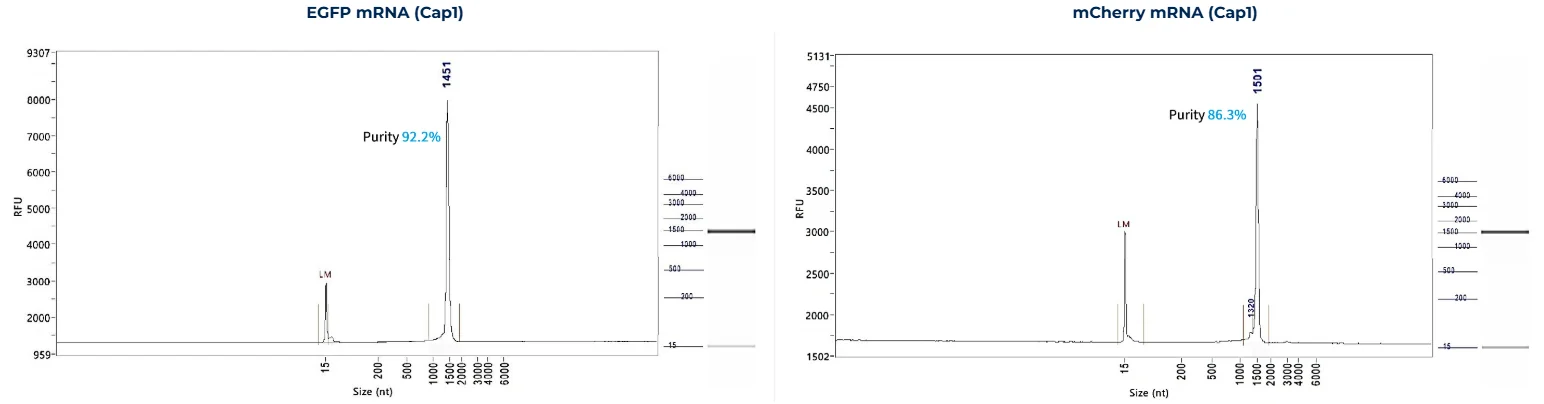
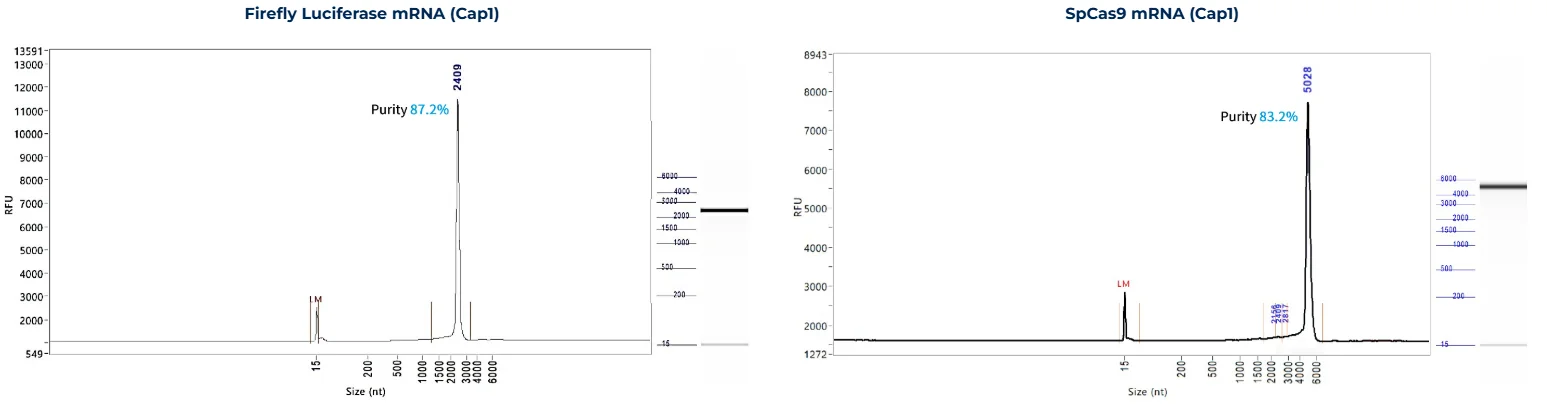
PackGene’s mRNA production demonstrates a size range within ±30% of the target size and a purity level exceeding 80%. Maintaining a ±30% size range ensures that the mRNA is appropriately sized for its intended application and maintains consistency across different sample batches. A purity level above 80% reflects a high concentration of mRNA with minimal contaminants, such as genomic DNA, RNA degradation products, and residual synthesis reagents, which could otherwise compromise the efficacy and safety of the mRNA in downstream applications.
These measurements are performed using the Agilent 5200 CE System, a highly sensitive and precise instrument designed for analyzing nucleic acids, including mRNA.
Application

Vaccine Development

Therapeutics

Cellular Programming
mRNA vaccines have gained widespread attention during the COVID-19 pandemic, with vaccines from Pfizer-BioNTech and Moderna receiving emergency use approval. These vaccines work by delivering a small piece of mRNA into the body, which instructs cells to produce a viral protein. The immune system identifies this protein as foreign and triggers an immune response, priming the body to fight the actual virus if encountered in the future.
mRNA also holds potential as a treatment for cancer, genetic disorders, neurodegenerative diseases, and more. In this approach, mRNA is used to introduce new genes into cells, either replacing or supplementing faulty genes responsible for diseases. This technique has shown promise in preclinical studies and is currently being evaluated in clinical trials.
mRNA can also be used to reprogram cells, transforming them into different cell types. This technique has the potential to revolutionize regenerative medicine by enabling the repair or replacement of damaged tissues or organs with healthy cells.
Publications
View more
- Aramesh, M., Yu, D., Essand, M., & Persson, C. (2023). Nanotopography boosts cellular uptake by inducing macropinocytosis. bioRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2023.12.20.571698
- Fekete, S., Imiołek, M., & Lauber, M. (2024, July 11). Advantages of sequential or bracketed injection methods to improve the chromatographic analysis of biotherapeutics. LCGC International – Separation Science News &Amp; Chromatography Insights. https://www.chromatographyonline.com/view/advantages-of-sequential-or-bracketed-injection-methods-to-improve-the-chromatographic-analysis-of-biotherapeutics
- Zhang, J., Li, C., Liu, Y., Liao, R., He, D., Xu, L., Chen, T., Xiao, Q., Luo, M., Chen, Y., Li, Y., Zhu, H., Rosenecker, J., Ding, X., Pei, S., & Guan, S. (2025). Airway applied mRNA vaccine needs tailored sequence design and high standard purification that removes devastating dsRNA contaminant. Molecular Therapy. https://doi.org/10.1016/j.ymthe.2025.05.024
GMP mRNA Manufacturing — From Development to Clinical Readiness
PackGene offers a comprehensive GMP mRNA manufacturing platform, supporting your journey from early development to clinical production. With advanced facilities and a robust quality management system, we ensure every batch meets the highest standards of purity, consistency, and regulatory compliance.
Key Features
End-to-end capability
From plasmid construction and mRNA synthesis to formulation development, fill and finish, and full documentation.
Seamless scale-up
Smooth transition from Process Development (PD) to cGMP manufacturing for research and clinical phases.
Customised process optimisation
Tailored workflows to maximise mRNA yield, stability, and reproducibility.
Global regulatory compliance
Operates under a QMS aligned with FDA, EMA, and NMPA standards.
Comprehensive analytical support
Includes in-process control, release testing, and stability studies for full traceability.
Regulatory-ready quality
Delivering GMP-grade mRNA materials suitable for IND filing and preclinical studies.
What We Offer
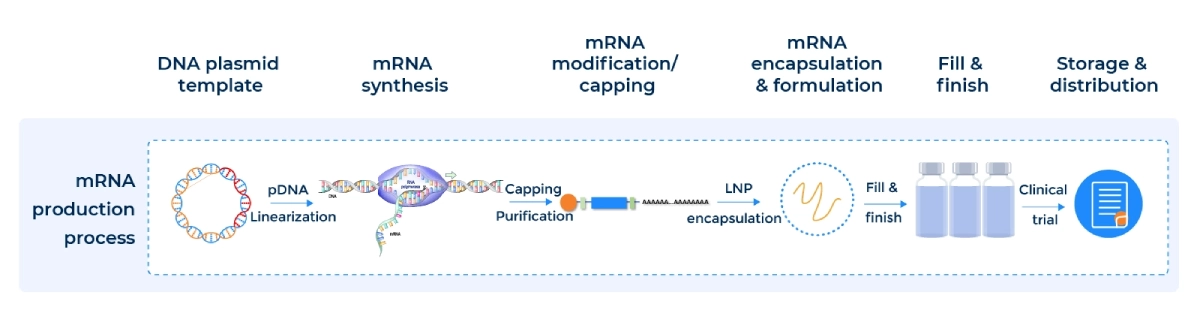
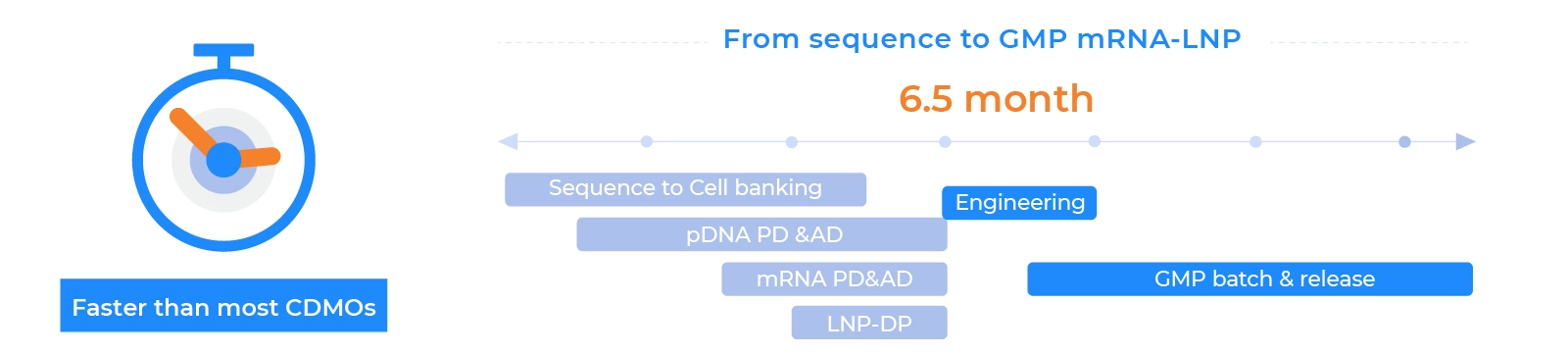
For all your mRNA manufacturing needs. Our streamlined process ensures ease in your CMC
Product Quality and Speed

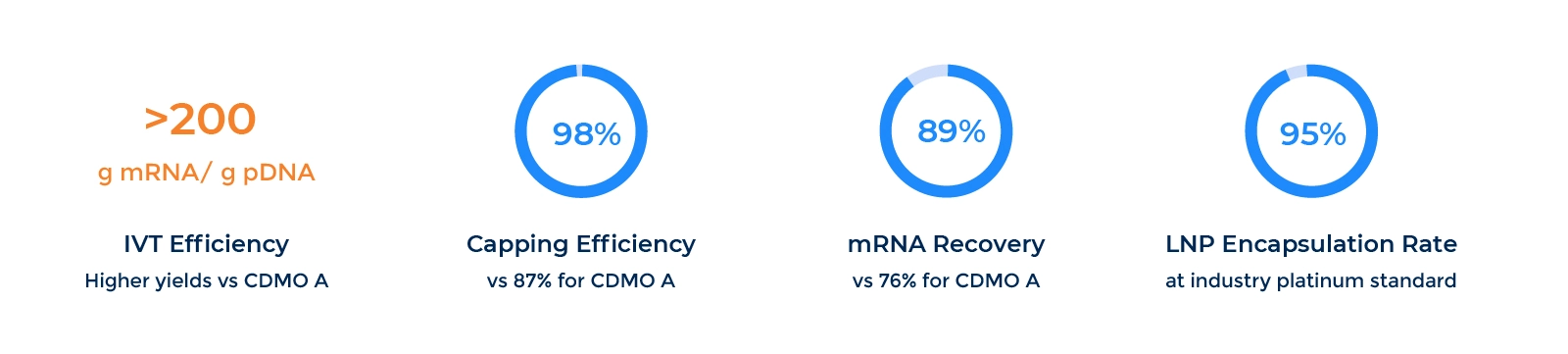
Comprehensive Analytical Panel
Packgene’s comprehensive analytical panel ensures every batch of mRNA meets the highest standards of purity, integrity, and consistency. Through in-process control, release testing, and stability studies, we help you achieve data you can trust for both research and GMP applications.
mRNA Analysis Includes:
- pH
- Content
- Sequence
- Purity
- mRNA Integrity
- Capping Efficiency
- Poly(A) Tail Length
- Residual pDNA
- Residual Protein
- Residual NTPs
- dsRNA Content
- Residual Solvents
- Bioburden
- Endotoxin
With advanced analytical tools and strict quality oversight, Packgene monitors each step of the mRNA production process to identify and resolve potential issues early. Their commitment to precision testing guarantees the accuracy, reproducibility, and regulatory readiness of your mRNA products.
GMP Documentation & Traceability
PackGene maintains complete documentation to ensure full regulatory compliance and consistent product quality. Every stage of mRNA manufacturing is traceable through well-structured project, operation, and quality records — supporting smooth transitions from R&D to clinical production.
| Project Document | Operation Document | Quality Document |
|---|---|---|
| Enquiry Assessment | SOPs | Specification |
| Manufacture Summary | Goods Receiving Record | Material and Product Test |
| Packaging Summary | Work Order | CoA |
| Delivery Summary | Batch Record | Batch Record Review and Release |
| Agreement and Contract | Shipping Request | Stability Data |
| Distribution Record | Validation Report | |
| Logbook | Supplier Qualification | |
| Environment Monitor |
Frequently Asked Questions
Can PackGene perform quality testing on customer-supplied mRNA?
Yes, Packgene can perform quality testing on mRNA provided by customers. Please refer to the mRNA QC tests we offer here.
What 5’ cap does PackGene offer?
We provide Cap1 capping through co-transcriptional capping by default. Alternatively, enzymatic capping for Cap1 is available at an additional cost.
What is the length range for PackGene’s mRNA production?
We can produce mRNA ranging from 400 bp to 10 kb. If you require mRNA beyond this length, please reach out to our technical support team, as special protocols may be needed.
How should I store my mRNA?
mRNA can be stable for at least 2 years when stored properly at -80°C and if repeated freeze-thaw cycles are avoided. Stability can vary based on the mRNA length, but we’ve tested mRNA to remain stable through 30 freeze-thaw cycles. For long-term storage, -80°C is ideal, while -20°C works for short-term storage.
How do you purify mRNA?
For RUO-grade customized mRNA, we use LiCl precipitation for quantities under 1 mg. For larger amounts (>1 mg) or off-the-shelf mRNA, we use HPLC-based Oligo dT purification to enhance RNA purity.
Are you provide mRNA codon optimization?
Yes, we offer codon optimization services. Please contact our technical support team for further details.
Trusted mRNA Partner in Southeast Asia
PackGene is the trusted partner for researchers advancing gene therapy and molecular biology across Southeast Asia — including Singapore, Thailand, Malaysia, and Indonesia. With over 1,000 global clients and 50,000+ successful projects delivered, PackGene combines a decade of viral vector and mRNA manufacturing expertise with a 120,000 sq ft GMP-compliant facility that meets FDA, EMA, and NMPA standards.
From research-grade to GMP-ready mRNA production, PackGene delivers speed, precision, and reliability that empower scientists and biotech innovators to move seamlessly from discovery to clinical translation.
Together with PackGene, we are committed to breaking barriers in gene therapy by providing comprehensive and cost-effective AAV, lentivirus, mRNA & LNP and plasmid solutions.
Contact us today to learn how our custom mRNA Production and Manufacturing Services can elevate your research to the next level.
Want To Inquire About The Services?
Contact Us

THE ATLANTIS BIOSCIENCE DIFFERENCE Discover Translational Solutions To Advance From Bench to Bed
GET SUPPORT Whenever You Need It

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotation.





