Overview of Human TNFa (Tumor Necrosis Factor Alpha) ELISA Kit
| Product name: | Human TNFa(Tumor Necrosis Factor Alpha) ELISA Kit |
| Reactivity: | Human |
| Alternative Names: | TNF Alpha; DIF; TNF-A; TNFSF2; Cachectin; Tumor Necrosis Factor Ligand Superfamily Member 2 |
| Assay Type: | Sandwich |
| Sensitivity: | 6.5 pg/mL |
| Standard: | 1000 pg/mL |
| Range: | 15.63-1000 pg/mL |
| Sample Type: | serum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids |
| Assay Length: | 3.5h |
| Research Area: | Cytokine;Tumor immunity;Infection immunity; |
| Test principle: | The test principle applied in this kit is Sandwich enzyme immunoassay. The microtiter plate provided in this kit has been pre-coated with an antibody specific to Tumor Necrosis Factor Alpha(TNFa). Standards or samples are added to the appropriate microtiter plate wells then with a biotin-conjugated antibody specific to Tumor Necrosis Factor Alpha(TNFa). Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. After TMB substrate solution is added, only those wells that contain Tumor Necrosis Factor Alpha(TNFa), biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450nm ± 10nm. The concentration of Tumor Necrosis Factor Alpha(TNFa) in the samples is then determined by comparing the OD of the samples to the standard curve. |
[accordions]
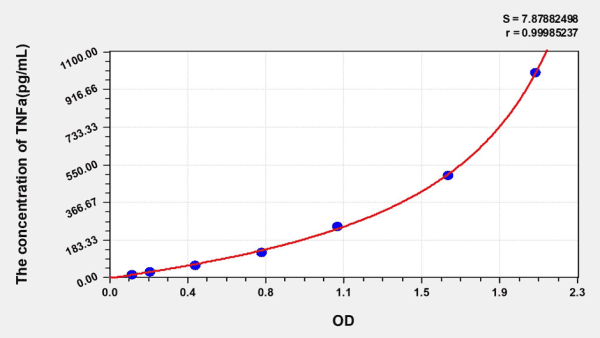
[accordion title= “Standard Curve“]
| Concentration (ng/mL) | OD | Corrected OD |
| 1000.00 | 2.214 | 2.086 |
| 500.00 | 1.784 | 1.656 |
| 250.00 | 1.243 | 1.115 |
| 125.00 | 0.872 | 0.744 |
| 62.50 | 0.546 | 0.418 |
| 31.25 | 0.325 | 0.197 |
| 15.63 | 0.240 | 0.112 |
| 0.00 | 0.128 | 0.000 |
[/accordion]
[accordion title= “Precision“]
Intra-assay Precision (Precision within an assay): CV%<8% Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision. Inter-assay Precision (Precision between assays): CV%<10% Three samples of known concentration were tested in forty separate assays to assess inter-assay precision. [/accordion] [accordion title= "Recovery“]
Matrices listed below were spiked with certain level of recombinant TNFa and the recovery rates were calculated by comparing the measured value to the expected amount of TNFa in samples.
| Matrix | Recovery range | Average |
| serum(n=5) | 83-95% | 89% |
| EDTA plasma(n=5) | 88-102% | 95% |
| Heparin plasma(n=5) | 79-97% | 88% |
[/accordion]
[accordion title=”Linearity“]
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of TNFa and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Matrix | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 87-96% | 91-101% | 87-98% | 89-103% |
| EDTA plasma(n=5) | 84-94% | 85-103% | 94-105% | 92-101% |
| Heparin plasma(n=5) | 88-104% | 84-98% | 95-105% | 88-102% |
[/accordion]
[accordion title=”Publication“]
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8819543/
- https://www.sciencedirect.com/science/article/abs/pii/S0378111920304339
- https://www.sciencedirect.com/science/article/pii/S2667031321000038?via%3Dihub
- https://www.sciencedirect.com/science/article/pii/S0753332221008544
- https://www.sciencedirect.com/science/article/abs/pii/S002432052030597X?via%3Dihub
- https://www.spandidos-publications.com/10.3892/etm.2021.9776
- https://www.researchsquare.com/article/rs-910465/v1
[/accordion]