- Your cart is empty
- Continue Shopping
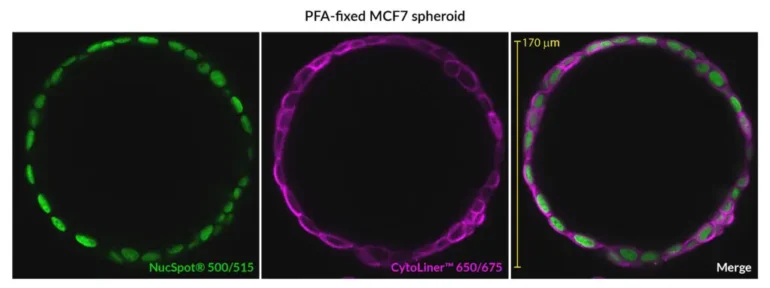
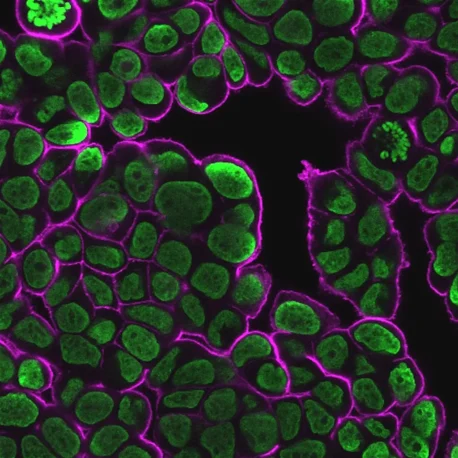
CytoLiner™ Fixed Cell Membrane Stains
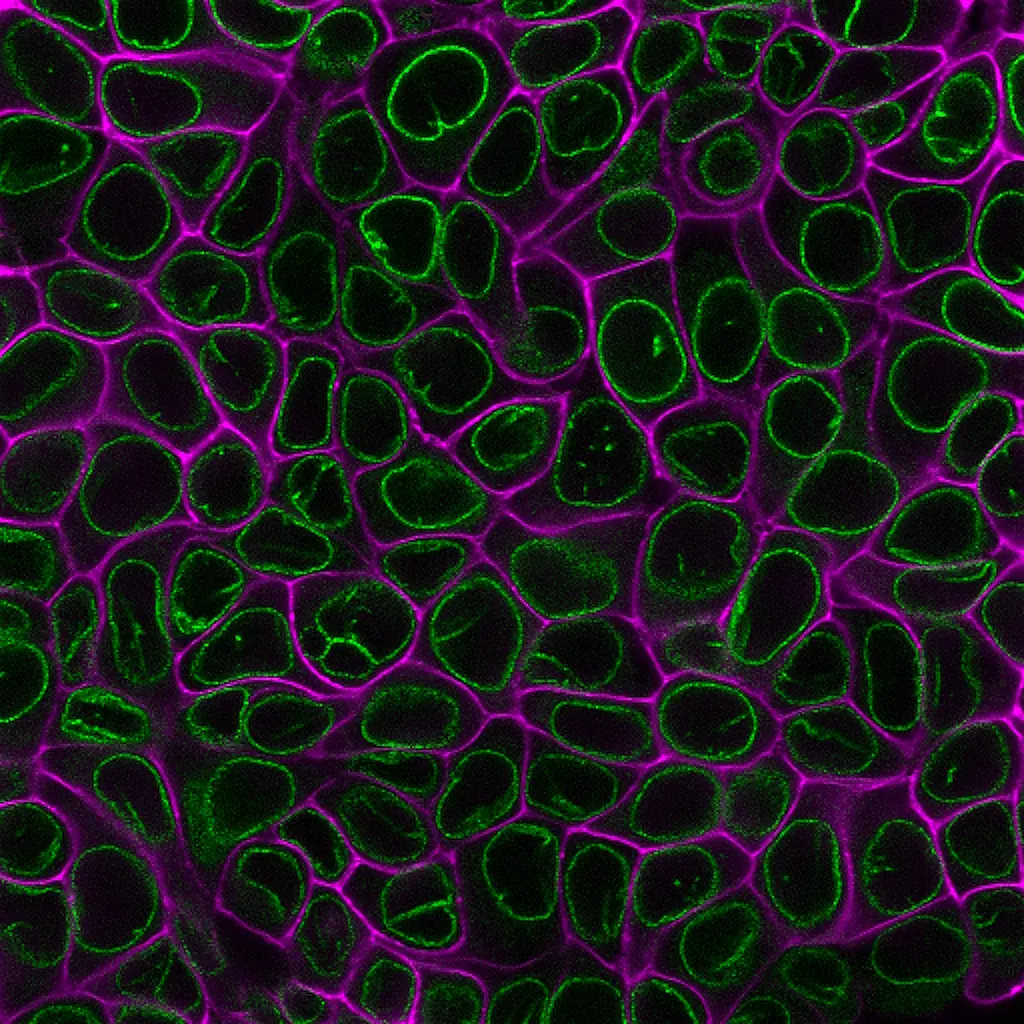
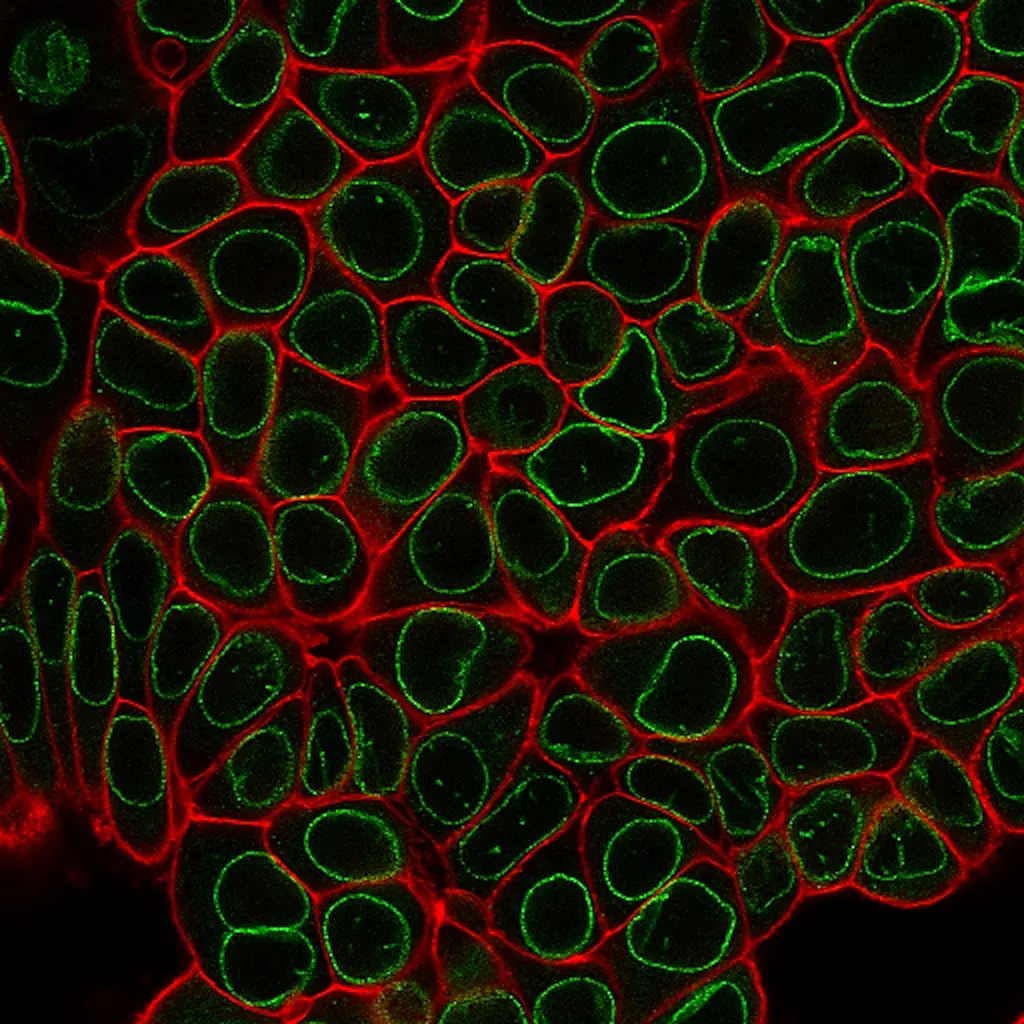
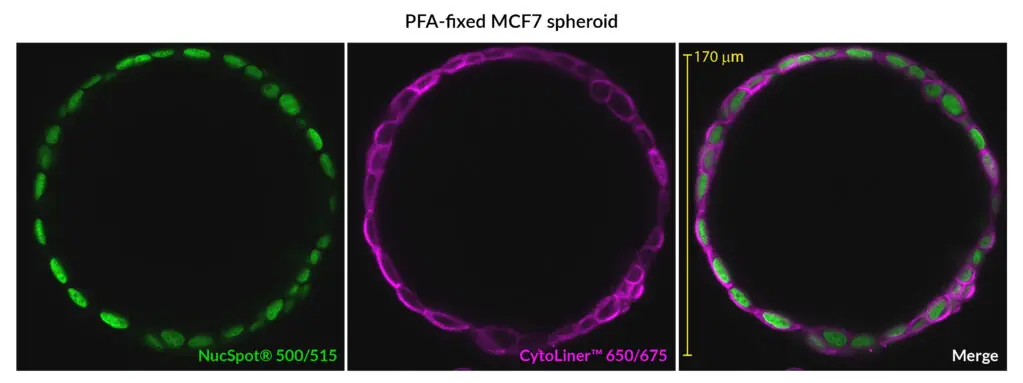
CytoLiner™ Fixed Cell Membrane Stains are novel lipophilic fluorescent dyes engineered for robust and consistent plasma membrane staining in formaldehyde-fixed cells. Available in six colours from blue to near-infrared, these dyes are optimised for microscopy and immunofluorescence workflows.
Novel Dyes for Reliable Membrane Labelling
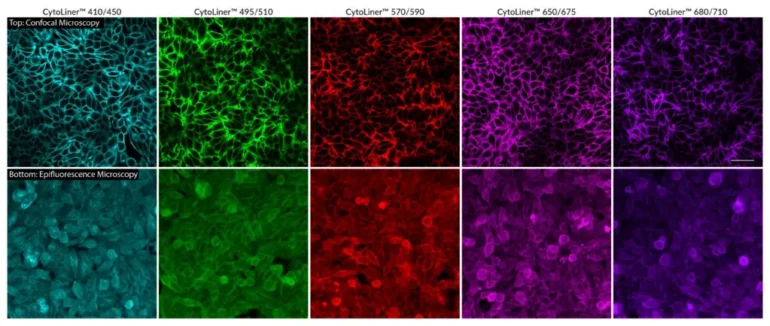
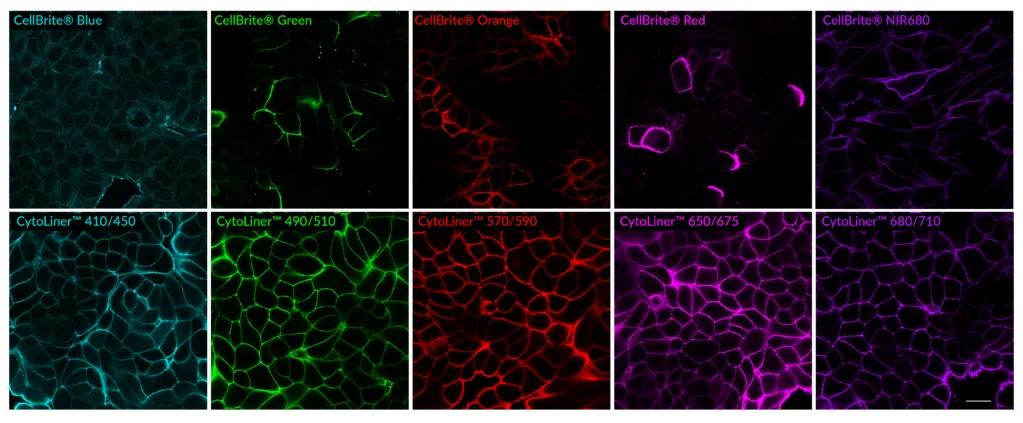
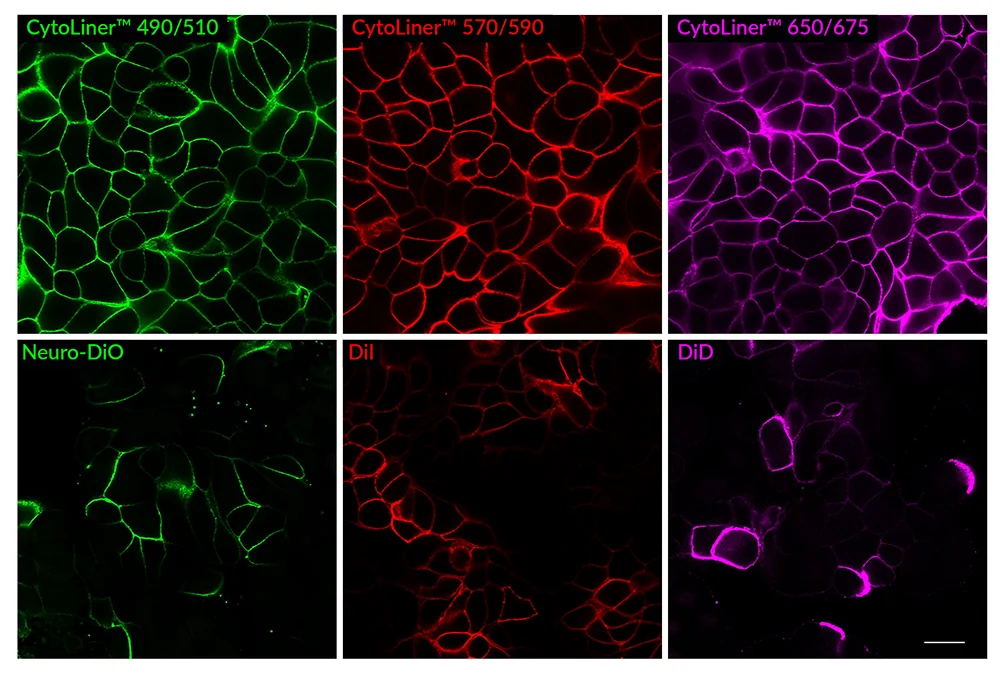
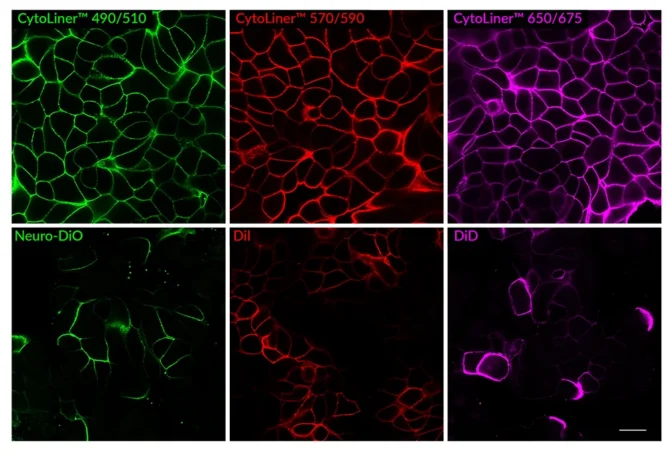
CytoLiner™ Fixed Cell Membrane Dyes represent a new generation of lipophilic fluorescent dyes specifically developed for selective plasma membrane staining in fixed and mildly permeabilised cells. Unlike traditional carbocyanine dyes such as DiI, which often show poor solubility and inconsistent staining, CytoLiner™ dyes are uniquely formulated for reliable and uniform membrane labelling in formaldehyde-fixed samples.
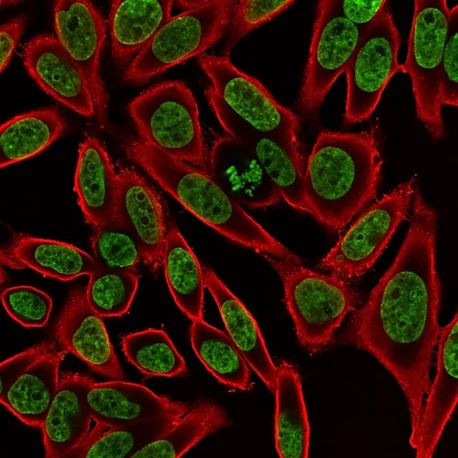
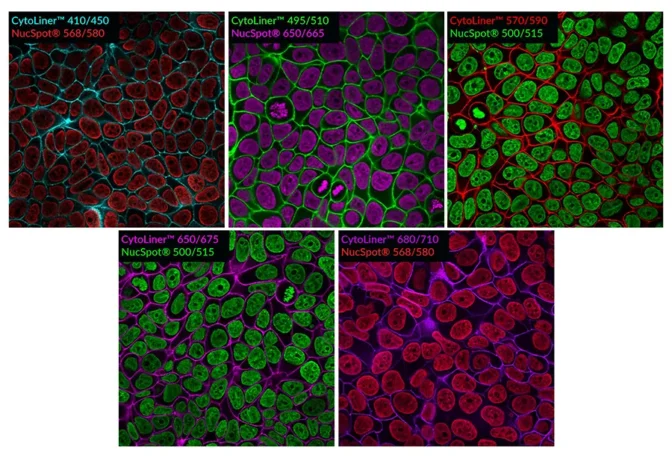
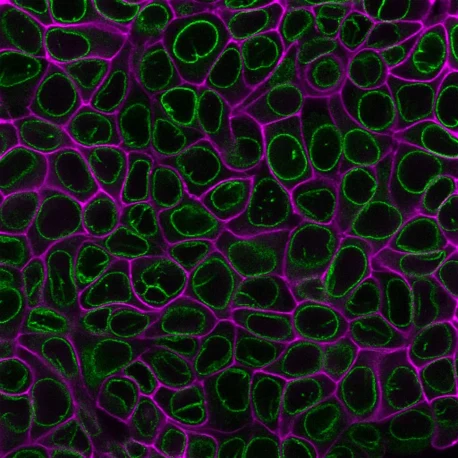
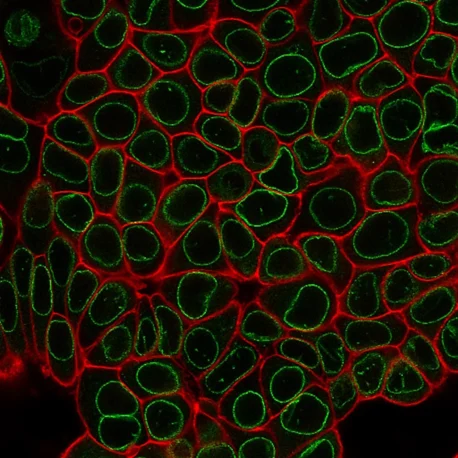
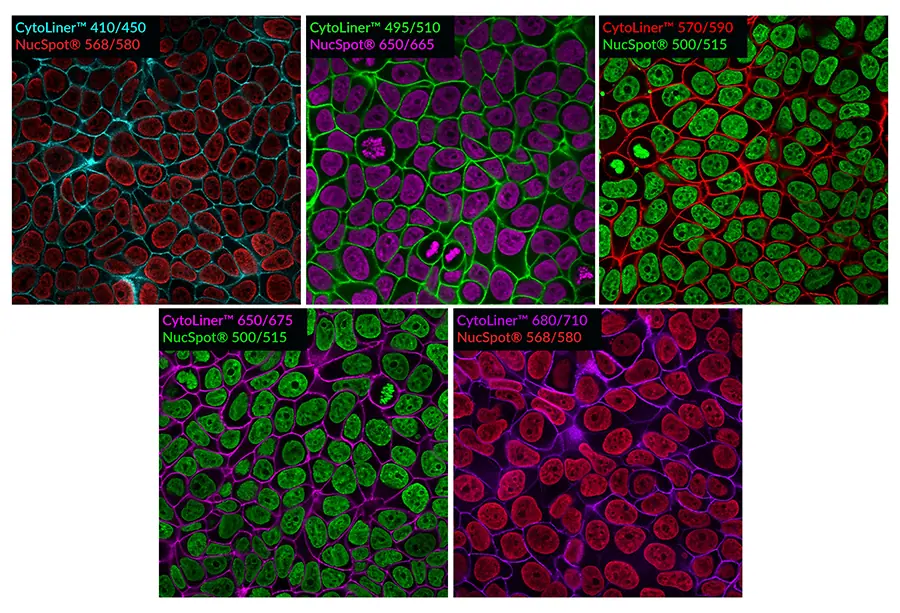
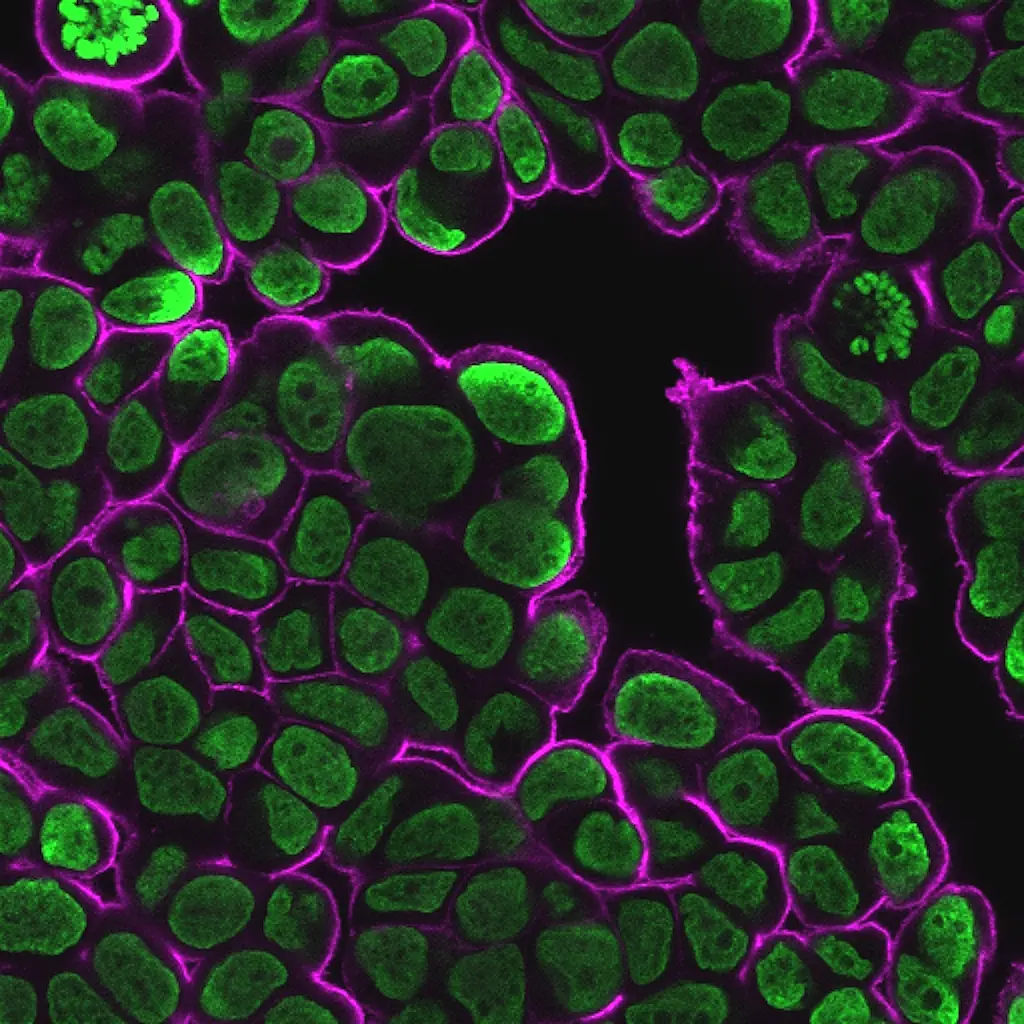
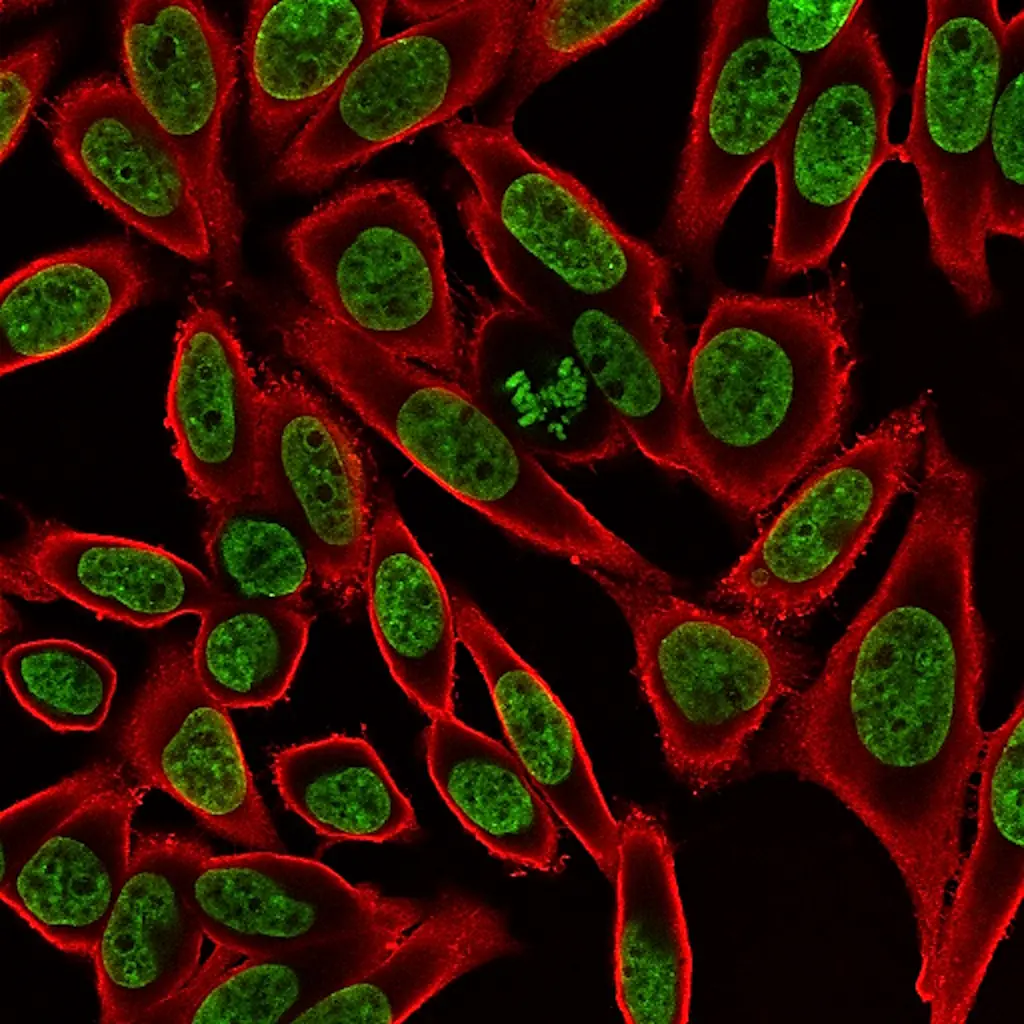
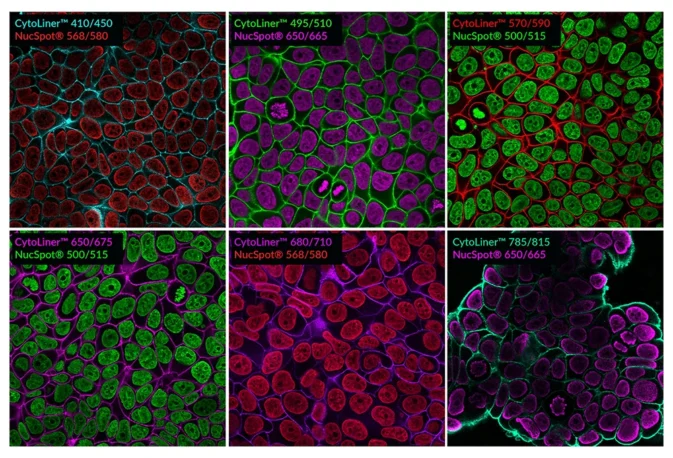
These dyes are compatible with immunofluorescence protocols, tolerating blocking agents, detergents, and antibody co-staining. They can also be used with poly-L-lysine coated cultureware and Transwell® supports, ensuring flexibility across a wide range of microscopy applications.
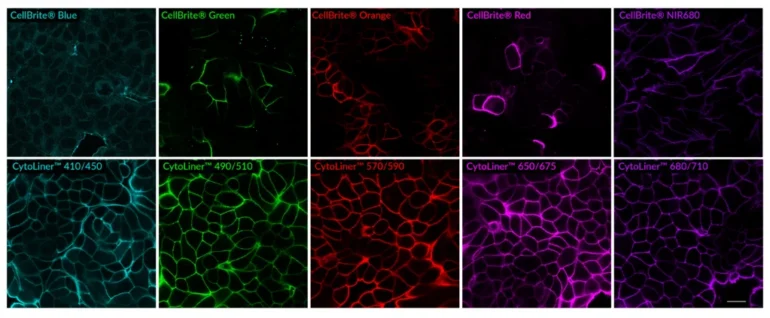
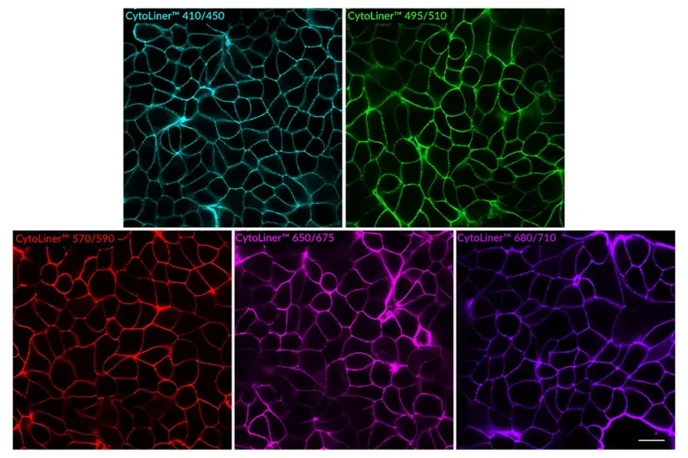
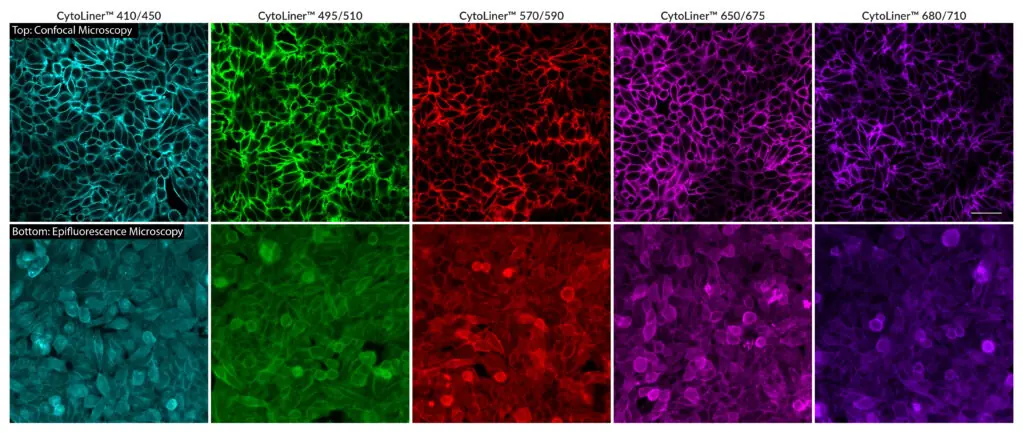
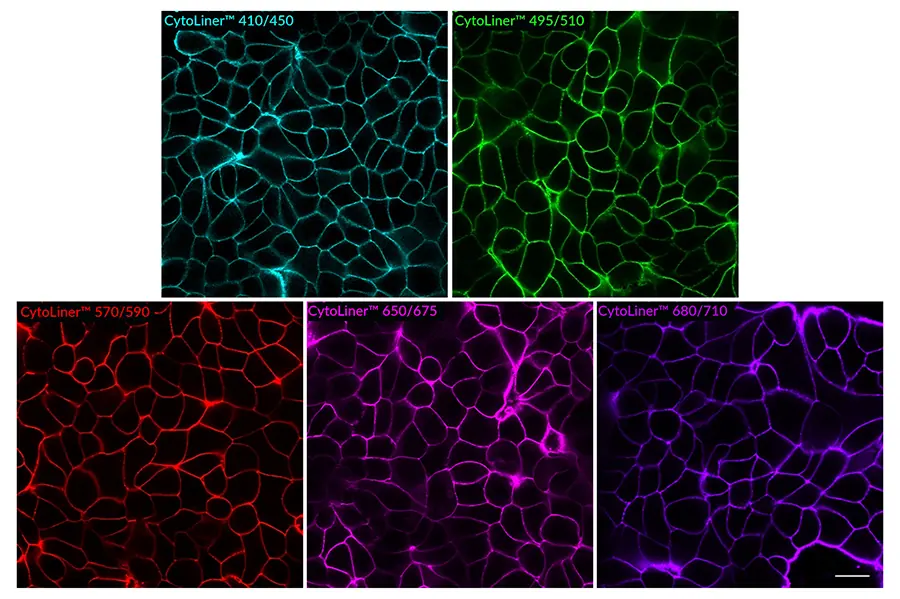
CytoLiner™ dyes are available in six excitation/emission pairs covering blue to near-IR detection channels, making them suitable for multicolour imaging panels.
Key Features
- Robust, consistent plasma membrane staining in formaldehyde-fixed cells
- Optimised for downstream antibody co-staining and multiplex imaging
- Compatible with poly-L-lysine coated plates and Transwell® inserts
- Works with mild detergent permeabilisation and blocking agents
- Available in six spectral variants (410/450 to 785/815 nm)
- Supplied as 500X dye in DMSO plus 100X staining buffer
Superior Staining Performance Over Classic Membrane Dyes
Suitable for Co-Staining with Antibodies and Other Probes
Kit Components:
- CytoLiner™ Fixed Cell Membrane Dye (500X in DMSO)
- CytoLiner™ Fixed Cell Staining Buffer (100X)
Notes: Biotium products are available only in Singapore and Thailand.
*Please leave us a message during checkout to indicate which kit you need, and our team will process your order accordingly.
Variation
| Variant | Excitation/Emission (nm) | Detection Channels | Cat. No. (1000 Labelings) | Cat. No. (250 Labelings) |
|---|---|---|---|---|
| CytoLiner™ 410/450 | 406/446 | DAPI/Pacific Blue™ | 30131 | 30131-T |
| CytoLiner™ 495/510 | 492/510 | FITC | 30132 | 30132-T |
| CytoLiner™ 570/590 | 573/592 | Cy®3/TRITC | 30133 | 30133-T |
| CytoLiner™ 650/675 | 647/674 | Cy®5 | 30134 | 30134-T |
| CytoLiner™ 680/710 | 682/707 | Cy®5.5 | 30135 | 30135-T |
| CytoLiner™ 785/815 | 787/819 | Alexa Fluor® 790 | 30140 | 30140-T |
Proven Performance