Noggin is a bioactive protein intended for use in cell culture applications. Noggin protein which bind to ligands of the TGF-β family and regulate their activity by inhibiting their access to signaling receptors. Noggin was originally identified as a BMP-4 antagonist whose action is critical for proper formation of the head and other dorsal structures.
Sequence:
MQHYLHIRPAPSDNLPLVDLIEHPDPIFDPKEKDLNETLLRSLLGGHYDPGFMATSPPEDRPGGGGGAAGGAEDLAELDQLLRQRPSGAMP
SEIKGLEFSEGLAQGKKQRLSKKLRRKLQMWLWSQTFCPVLYAWNDLGSRFWPRYVKVGSCFSKRSCSVPEGMVCKPSKSVHLTVLRWRC
QRRGGQRCGWIPIQYPIISECKCSC with polyhistidine tag at the C-terminus
Source:
Escherichia coli
Animal-free reagent and laboratory
Manufactured and tested under GMP guideline
Endotoxin level:
<0.1 EU per 1 μg of the protein by the LAL method.
Activity:
Measure by its ability to inhibit BMP-4-induced alkaline phosphatase production by ATDC5 cells.
The ED50 for this effect is <0.05 μg/mL in the presence of 50 ng/mL of recombinant human BMP-4.
Purity:
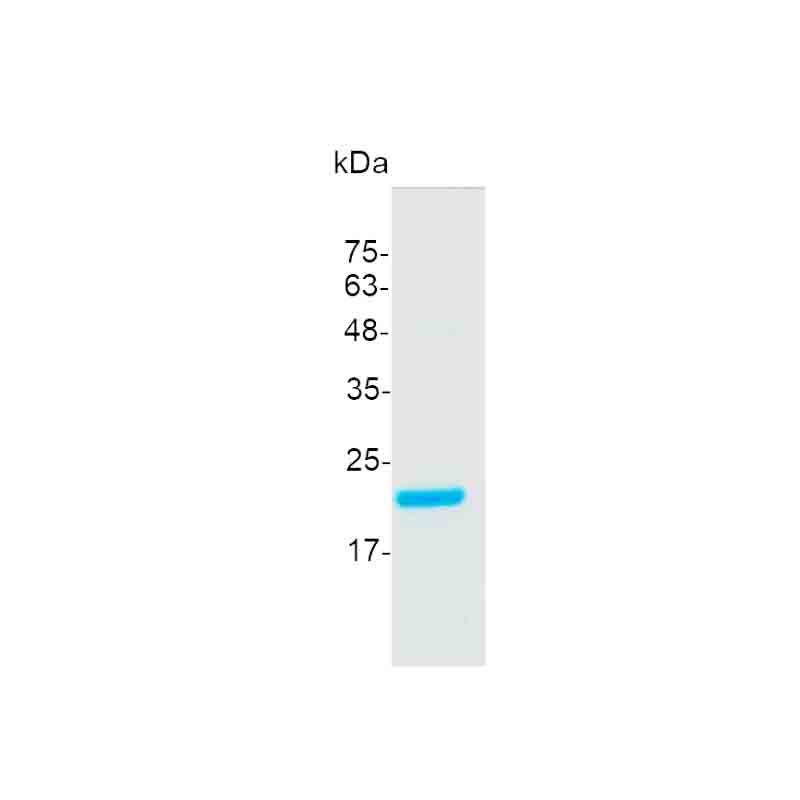
>98% as determined by SDS-PAGE. Purified by Ni-NTA chromatography.
Formulation:
The protein was lyophilized from a solution containing 1X PBS, pH 7.4.
Reconstitution:
It is recommended to reconstitute the lyophilized protein in sterile H2O to a concentration not less than 100 μg/mL and incubate the stock solution for at least 20 min to ensure sufficient re-dissolved.
Storage:
Lyophilized protein should be stored at -20°C. This product is stable for one year upon receipt, when handled and stored as instructed. Upon reconstitution, protein aliquots should be stored at -20°C or -80°C. Avoid repeated freeze/thaw cycles.
Note:
Please use within one month after protein reconstitution.
Specification:
Croyez GMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP-certified facility.
The processes include:
● Testing and traceability of raw material
● Records of the maintenance and equipment calibration
● Personnel training records
● Batch-to-batch consistency
● Documentation of QA control and process changes
● Manufactured and tested under an ISO 13485:2016 certified quality management system
● Stability monitor of product shelf-life
Reference:
1. Krause, C. et al. (2011) Int J Biochem Cell Biol. 43,4: 478-81.
2. Que, J. et al. (2006) Differentiation. 74,7: 422-37.