Product Description
Our cGMP AB serum delivers the best in safety and consistency.
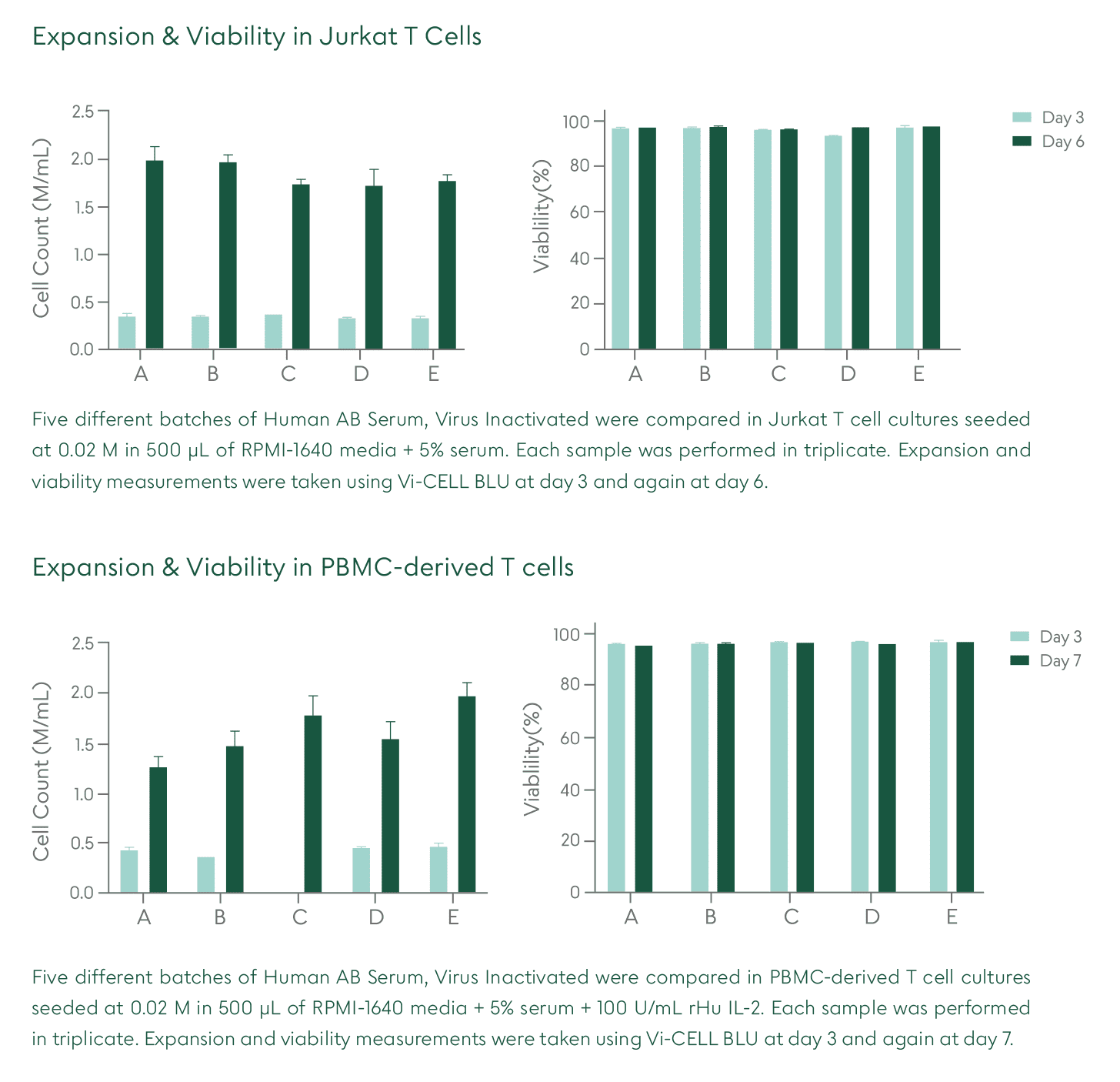
Akron’s Human AB Serum, Converted from Octaplas®, Pooled Plasma (Human), Xeno-Free, Virus Inactivated (viHABS) Closed System Solutions™ (CSS) is manufactured, tested, and released following relevant cGMP guidelines for blood-derived ancillary materials.
Raw material donor selection ensures that the product is devoid of antibodies for A and B blood antigens, therefore minimizing immune reactivity in cell culture. No animal-derived components are used in the manufacture of this product. Virus-inactivated plasma is used for all human-derived components. This product is considered a comparable and superior alternative to fetal bovine serum or other animal-based products. By leveraging Octaplas®, a pharmaceutically licensed virus inactivated and prion-ligand treated plasma, as a raw material, Akron is now offering a high quality, virus inactivated human AB serum product with greater batch-to-batch consistency and a unique safety profile.
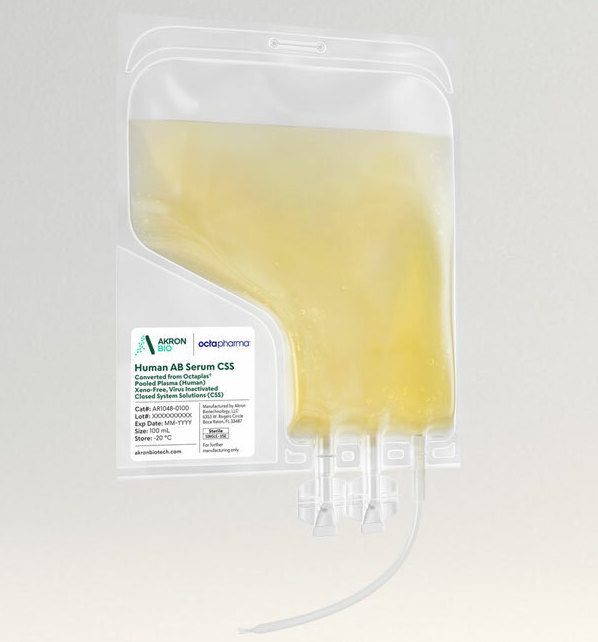
The viHABS CSS product is packaged in a sterile single-use bag with weldable tubing, allowing for easy incorporation into modern closed-system cell culture bioprocessing protocols. viHABS CSS increases safety and ease of use by allowing for the introduction of supplement material into culture media in a fully contained manner. The final product undergoes Endotoxin, Mycoplasma, and Sterility testing. See product Packaging features below.
Donor Eligibility
• Pharmaceutically licensed, pooled plasma from US licensed plasma donation centers
• Collection complies with WHO Technical Report Series 840: “The Collection, Fractionation, Quality Control, and uses of Blood and Blood Products
• Nucleic acid virus testing (NAT) during multiple stages of manufacturing (HIV, B19, HAV, HBV, HCV, HEV)
• Donor screening and virus testing per 21 CFR 610.40
• Type AB donations devoid of antibodies for A and B blood antigens
Plasma Source
• Octaplas® is an FDA-approved, sterile, pyrogen-free, frozen, S/D treated, prion-ligand treated, pooled human plasma
• Solvent Detergent (S/D) treatment for inactivation of enveloped viruses
• Immune Neutralization for inactivation of certain non-enveloped viruses
• Affinity Chromatography intended to reduce prion proteins
• Sterile microfiltration minimizes the presence of bacteria and parasites
Manufacturing
• No animal-derived materials are used in the manufacture of this product
• Sterile microfiltration followed by aseptic filling
Packaging
• Sterile bag chamber – Ethylene-vinyl acetate (EVA) for inert bio-reactivity and increased flexibility
• Weldable polyvinyl chloride (PVC) 6” outlet tubing (2.5 mm ID x 4.1 mm OD) with female Luer adapter
• End of inlet tubing sealed using controlled radiofrequency or heat
• Two twist-off spike ports allowing for various attachments and adapters to fit your purpose
• Tubes and ports are welded into the bag chamber eliminating potential failure points
• All primary packaging is plasticizer free
• Primary packaging materials extensively validated, controlled, and qualified to ensure a consistent experience
Quality
• Relevant cGMP guidelines used in manufacture, testing, and release
– USP Chapter <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
– ISO 13485:2016, Medical devices – Quality management systems – Requirements for regulatory purposes
– ISO/TS 20399-1-3:2018, Biotechnology – Ancillary materials present during the production of cellular therapeutic products
Stability
• Under a long-term stability program
• Store at -20 °C
• Transport on dry ice
• Avoid repeated freeze-thaw cycles
For Use Statement
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.