The Best of Safety and Consistency
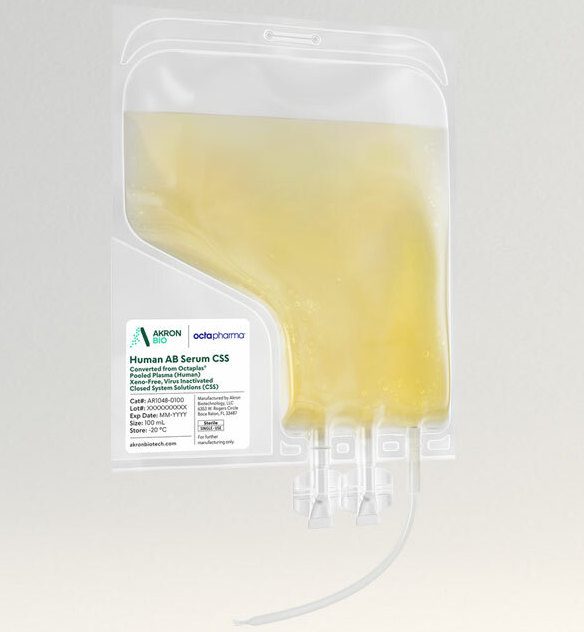
Akron’s Human AB Serum, Converted from Octaplas®, Pooled Plasma (Human), Xeno-Free, Virus Inactivated (viHABS) Closed System Solutions™ (CSS) is manufactured, tested, and released following relevant cGMP guidelines for blood-derived ancillary materials.
Raw material donor selection ensures that the product is devoid of antibodies for A and B blood antigens, therefore minimizing immune reactivity in cell culture. No animal-derived components are used in the manufacture of this product. Virus-inactivated plasma is used for all human-derived components. This product is considered a comparable and superior alternative to fetal bovine serum or other animal-based products. By leveraging Octaplas®, a pharmaceutically licensed virus inactivated and prion-ligand treated plasma, as a raw material, Akron is now offering a high quality, virus inactivated human AB serum product with greater batch-to-batch consistency and a unique safety profile.
The viHABS CSS product is packaged in a sterile single-use bag with weldable tubing, allowing for easy incorporation into modern closed-system cell culture bioprocessing protocols. viHABS CSS increases safety and ease of use by allowing for the introduction of supplement material into culture media in a fully contained manner. The final product undergoes Endotoxin, Mycoplasma, and Sterility testing. See product Packaging features below.