ActSep®CD3/CD28 Separation & Activation Magnetic Beads
- FDA DMF filed: 038124
- High stability and safety, low endotoxin.
- 4.5um superparamagnetic beads, artificial antigen presenting particles.
- High efficiency of cell separation and activation. Easy removal of magnetic beads, easy operation.
Product Description
ActSep®CD3/CD28 Separation & Activation Magnetic Beads are suitable for separation of human CD3+T cells, providing a simple method for activating and expanding human T cells without the need for antigen presenting cells and antigens. By coupling anti-human CD3 and anti-human CD28 antibodies on magnetic beads, the primary and co-stimulatory signals needed to regulate T cell activation and expansion are provided. The beads are suitable for human T cell separation, activation and expansion, CAR-T and other T cell culture applications.
Catalog Number : GMP-TL603
Content : 1mL
Consistence : 2×108 beads / mL
Endotoxin : <0.5 EU/mL
Reactive Species : human
Validity Period : 24 months
Storage Temperature : 2~8C
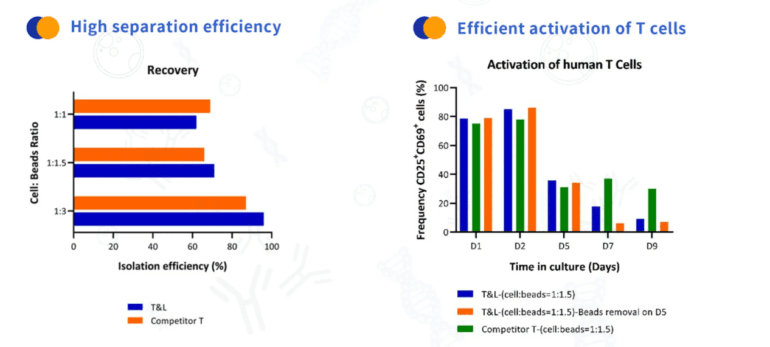
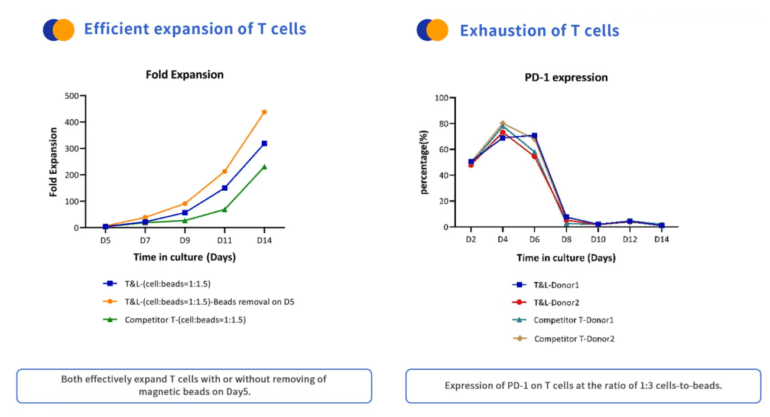
Product Performance
Protocol
1. PBMC cell treatment:
1.1 Resuspend human PBMC cells in PBS buffer containing 1% HSA and determine the percentage of CD3+ T cells in the sample by flow cytometry.
1.2 According to the CD3 positive rate of PBMC cells, adjust the density of CD3+ T cells to 1×107 cells/mL with PBS buffer containing 1% HSA.
2. Wash ActSep® CD3/CD28 separation & activation magnetic beads:
2.1 Resuspend the magnetic beads (vortex for more than 30 seconds, or tilt and rotate for 5 minutes).
2.2 According to the number of magnetic beads and CD3+ T cells, the recommended bead: T cell ratio is 1:1 ~ 3:1 (In this interval, with the increase of the amount of magnetic beads, the cell separation efficiency will be improved. In practical applications, customers can adjust the ratio according to the actual situation). Transfer the magnetic beads of a certain volume into the centrifugal tube.
2.3 Add 1 mL of PBS buffer containing 1% HSA, then resuspend and vortex.
2.4 Place the tube on a magnet for 1 minute, then discard the supernatant. Be careful not to inhale the magnetic beads.
3. Separation and activation CD3 + T cells:
3.1 Add PBMC adjusted CD3+ T cell density to the above washed magnetic beads in proportion, and mix well.
3.2 Place the above centrifuge tube on a sample mixer with a rotating speed of 15~30 rpm and incubate at room temperature for 30 min.
3.3 Put the above centrifuge tube on the magnet for 1 min and collect the supernatant. Count the cells and stain with anti-CD3 antibody for flow cytometric analysis to calculate separation efficiency in an indirect way.
3.4 Resuspend the mixture of magnetic beads and cells with a complete medium containing 200 IU/mL rh IL-2 (Customers can make adjustment according to their actual experimental conditions, and determine whether to use autologous plasma according to experimental needs), and adjust the cell density to 0.8~1.5×106/mL.T
3.5 Place culture vessels in a cell culture container, incubate at 37°C, 5% CO2 for 48 hours. Take samples, count and test for activation efficiency after removal of magnetic beads (CD69+CD25+ percentage).
4. Cell expansion and culture: Observe the cell status and supplement expansion medium regularly. When T cell density is > 2.5 × 106 cells/mL or medium turns yellow, dilute the cells to approximately 0.5~1×106 T cells/mL. Cells can be harvested after culturing for an appropriate length of time (usually 12-14 days).
Notice:
1. After cell expansion, the cell suspension in the culture vessel should be gently blown periodically to allow adequate dispersion of the magnetic beads and cells.
2. During the culture process, the cells could be counted every 2 to 3 days, and the magnetic beads should be removed before cell counting. The magnetic beads removal process: the cells in the culture vessels were evenly suspended and sampled into the centrifuge tube, placed on the magnet, left for 1 min, and then transfer the supernatant to a new centrifuge tube.
3. T Cells were harvested after removal of magnetic beads after being cultured for a suitable number of days.
4. The purity, phenotype and activation state of T cells could be detected by flow cytometry during cell culture.
Reference Publications
Pan, J., Tan, Y., Wang, G., Deng, B., Ling, Z., Song, W., Seery, S., Zhang, Y., Peng, S., Xu, J., Duan, J., Wang, Z., Yu, X., Zheng, Q., Xu, X., Yuan, Y., Yan, F., Tian, Z., Tang, K., Zhang, J., … Feng, X.
Donor-Derived CD7 Chimeric Antigen Receptor T Cells for T-Cell Acute Lymphoblastic Leukemia: First-in-Human, Phase I Trial.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology vol. 39,30 (2021): 3340-3351. doi:10.1200/JCO.21.00389