- Your cart is empty
- Continue Shopping

싱가포르의 식량안전보장 촉진 위한 대체 단백질 사용법에 대한 고찰
싱가포르 – 점심시간 때의 싱가포르 호커 먹거리 골목(Hawker stall)에서, 젊은 농부들이 자신들이 생산을 도와 만들어 자랑스럽다고 말한 닭고기 사테를 먹기 위해 앉았습니다.

If you’re thinking to yourself ‘How is cultured meat made?‘ you’ve landed on the right page. Lab-grown meat starts with a key ingredient: cells. The cells used to produce cultured meat must adhere to a type of structural support, known as a scaffold, to be able to produce thick meat product structures like steak, patty and sashimi.
However, there is no single ‘recipe’ for how cultured meat gets developed. Scientists from different research domains need to derive technologies from tissue engineering, regenerative medicine, and biomaterials to form a complex structure of lab-grown meat. Getting cells to look, feel & taste like meat is not an easy job, having a good scaffold for your cells is one of the key components researchers focus on when designing & crafting their cultured meat.
Stem cells are often used to produce cell-based meat. It is the starting material in cultured meat production used to create muscle cells which eventually grow to form meat products. The source of stem cells needed for your meat of interest is often found in the ECM. The ECM are non-cellular components that provide support and structures for cells in the body. It provides a supportive and scaffold-like structure to support the growth of muscle cells by helping cells to organize, differentiate & form into a tissue-like structure that resembles meat.
ECM mimics the natural environment that cells would experience within the body to ensure the growth and survival of cells throughout the production process. Hence, different ECM stiffness has an important influence on the downstream processing such as polarity, migration and developmental landmarks of cells. ECM has a significant impact on the regulation of stemness and differentiation across different types of stem cells (iPSC, MSC, Satellite and adipogenic stem cells). Other than the mimicry of ECM stiffness to an optimal physiological-like state, the ECM protein composition together paired with variations of cell culture media can drive the initiation phase for the creation of structural meat.
Stemness:
the ability of stem cells to maintain their properties & continue to differentiate into the desired tissue type
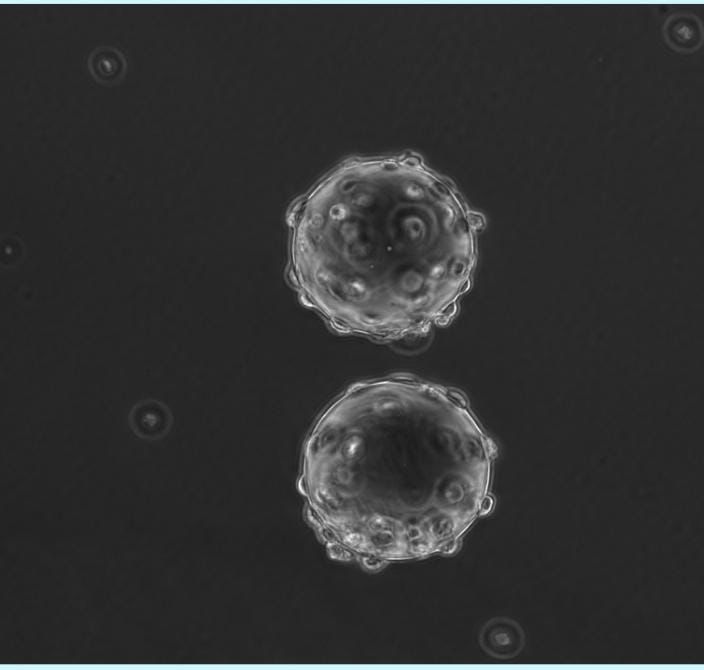
Microcarriers are bead-like structures that act as an attachment sites for cells helping cells to grow and maintain viability and health in the production process. It is a commonly used scaffold for cultured meat production as it helps increase production efficiency by increasing the surface area available for cell growth which yields a high number of cells cultured in a specific volume.
It is usually made from materials like polystyrene, polyacrylamide, glass or dextran, andThe use of a microcarrier to produce cultured meat usually occurs in a large-scale bioreactor as it allows large-scale production of cells in a controlled environment for commercial applications. The cell grown on microcarriers can then be harvested and combined to form a tissue structure that resembles real meat. There is still room for further commercial use optimization of microcarriers for various cell types and cell lines. Some of the edible microcarriers that are available in the market and approved by the FDA include pectin, chitosan, gellan gum, gelatin, cellulose, starch, and alginate.
Scaffolds provide a 3D structure for cell attachment, growth and organization to form a structured and thick meat. The scaffold material is often designed to mimic the structure and mechanical properties of native tissues. Scaffold materials are categorized by their source of biomaterials into natural (vertebrate ECM, algae, agarose, chitosan, fungal mycelium or etc.), synthetic or hybrid. These natural materials have high biocompatibility, high degradability and low immunogenicity which allows strong cell adhesion motifs.
Some common synthetic biomaterials include Pluronic, poly(ethylene glycol) (PEG), polyglycolic acid (PGA), poly(2-hydroxy ethyl methacrylate) (PHEMA), and poly(acrylamide) which have been found to have poor support efficiency for cell adhesion.
Hydrogel is a type of hydrophilic 3D network of polymer chains used as a scaffold material. It is commonly used in 3D cell culture as well as 3D cultured meat production. Hydrogels can absorb large amounts of water and form a gel-like structure giving cells a 3D environment to grow. It can be customized to mimic different environmental stimuli for specific cell growth and determine cell fate.
The development of hydrogel for cultivated meat is challenging as a general scaffold is used for transplantation that allows neovascularization of tissue, but for cultivated meat, it is needed to support cell viability via perfusion within the bioreactor. Similarly to microcarriers, hydrogels can be functionalized with various molecules like growth factors or ECM components to further optimize cell growth.
When using hydrogels, parameters like pore size, shape, and roughness should also be considered. Hydrogel is an attractive scaffold material for much-cultured meat researchers as it is non-toxic, biocompatible and can be easily manufactured in large quantities.
3D bioprinting involves using a computer-aided design to print substrate layer-by-layer end product. It creates structures for cell-cultured meat by depositing cells or bio-ink to create a three-dimensional structure. There are several different types of bioprinting and explained below:
3D bioprinting can be combined with other scaffold technologies such as hydrogels or ECM to further support cell growth.
Decellularization is the process where scientists remove the cells and nucleic acid from a tissue while maintaining the native ECM to act as a scaffold. This is suitable for organ regeneration and can be applied to plant tissues, and to some animal tissue as well.
This method has been explored as a scaffold in cell-cultured meat production by removing cells from a piece of meat leaving behind the ECM. The ECM is then seeded with muscle cells or other cell types which will be cultured and eventually produce cell-cultured meat with mechanical and biochemical properties similar to native tissue. However, the decellularization process is complex and requires specialized equipment and expertise. Scalability & commercialization using this method is still in question.
Scaffolds are fabricated and cells are seeded on the scaffold. One drawback is it struggles to recapitulate tissue microstructure.
Aims to create a microscale that assembles a more complex structure
eg. 3D printing
There are various scaffold methods used by researchers working with cell-cultured meat. When it comes to commercialization, there might be some limitations in the scale-up process. From the types of scaffolds mentioned, the most expensive would be 3D bioprinting as it requires specialized equipment, and expertise, and can be time-consuming.
Ongoing research has been done to explore new and innovative scaffold technologies to create safe, sustainable and affordable cultured meat. Regardless of the methods chosen, the goal remains the same: to provide cells with a supportive and biologically relevant environment that promotes growth and yields high-quality cell-cultured meat products that suit consumers’ preferences.
Reference:
Cultivated meat scaffolding: Deep Dive: GFI. The Good Food Institute. (2021, August 6). Retrieved from
https://gfi.org/science/the-science-of-cultivated-meat/deep-dive-cultivated-meat-scaffolding/

Connect With Our Technical Specialist.

Request For A Quotation.

싱가포르 – 점심시간 때의 싱가포르 호커 먹거리 골목(Hawker stall)에서, 젊은 농부들이 자신들이 생산을 도와 만들어 자랑스럽다고 말한 닭고기 사테를 먹기 위해 앉았습니다.

Contact our Customer Care, Sales & Scientific Assistance

Consult and asked questions about our products & services

Documentation of Technical & Safety Data Sheet, Guides and more...