- Your cart is empty
- Continue Shopping
EV Staining Revolution: Move Beyond PKH and Carbocyanine Dyes with Cutting-Edge Alternatives
EV Staining Revolution: Move Beyond PKH and Carbocyanine Dyes with Cutting-Edge Alternatives
- Atlantis Bioscience
- Blog
- Reading Time: 5 minutes
Extracellular vesicles (EVs) are minuscule, membrane-bound structures that cells release into their environment. These vesicles act as cellular couriers, transporting proteins, DNA, and RNA molecules to other cells. Over the last decade, EVs have garnered significant attention due to their potential as natural carriers for complex cellular components and their involvement in various physiological and pathological processes.
Advanced Techniques for Understanding EV Functions
A multitude of in vitro and in vivo techniques are employed to delve into the biological functions of EVs. These include flow cytometry, confocal microscopy, and in vivo fluorescence detection methods. These techniques often rely on labelling EVs to facilitate their detection and analysis. Labelling strategies encompass optical (fluorescence, bioluminescence), nuclear, and magnetic resonance imaging (MRI) tracers, as illustrated in Figure 1. These advanced methods enable comprehensive insights into EV functions, interactions, and roles in physiological and pathological processes.
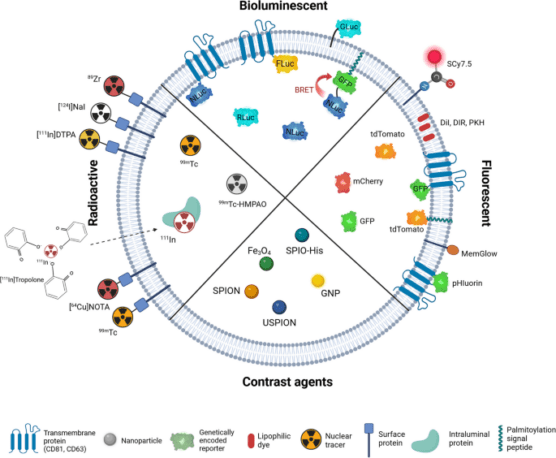
Figure 1: Strategies for labelling of exogenous extracellular vesicles.
Credit: Boudna A., Campos AD., et. al., https://doi.org/10.1186/s12964-024-01548-3
Reproduced under the Creative Commons license
Fluorescent Dyes for Exosome Labelling
Detecting and characterising small EVs poses a significant challenge in the field. Flow cytometry, however, is frequently utilised for its ability to perform rapid, high-throughput, multiparameter analysis. Various fluorescent probes are commonly employed to detect small EVs using flow cytometry, including:
1. Antibody-based Dyes:
These dyes exploit specific interactions with surface markers on small EVs, allowing for precise and targeted detection. These dyes are conjugated to antibodies that recognise and bind to distinct proteins on the EV membrane, facilitating their identification and characterisation. Notable surface markers include the tetraspanins CD9, CD63, and CD81. These proteins are highly enriched on the surface of EVs and are commonly used as markers for their detection. Antibody-based dyes targeting these tetraspanins provide specificity and sensitivity enabling the detailed analysis of EV populations, including their origin, composition, and potential functions. This targeted approach enhances the accuracy of flow cytometry in EV research, providing valuable insights into their roles in various physiological and pathological processes.
2. Membrane-Permeable Dyes:
Membrane-permeable dyes, such as carboxyfluorescein diacetate succinimidyl ester (CFSE), play a critical role in labelling and tracking EVs. CFSE is a lipophilic fluorescent dye that passively diffuses into cells and becomes highly fluorescent when intracellular esterases cleave its acetate groups. This reaction results in the dye covalently binding to amine groups in intracellular proteins, producing a stable and intense fluorescence signal. However, antigen expression and intracellular protein content vary depending on the cellular origin, leading to heterogeneity within a small EV population. Consequently, CFSE labelling may not be optimal for comparison across different samples. Additionally, the dye can leak from labelled cells, staining the surrounding environment and creating background noise that can obscure EV signals.
3. Lipophilic Dyes:
A variety of commercial lipid dyes are available, including PKH dyes and carbocyanine dyes (such as DiD, DiR, DiI). PKH dyes are highly fluorescent cell linkers that integrate into the cell membrane’s lipid bilayer. Common examples include PKH26 (red) and PKH67 (green). However, studies have shown that PKH dyes can bind non-specifically to other cellular components or form free dye aggregates, creating background noise and complicating the identification of labelled EVs. PKH dyes might also affect membrane–membrane fusion, fluidity of membrane proteins, membrane stiffness and EV size.
Carbocyanine dyes, such as DiO and DiI, are another class of lipophilic dyes used for EV labelling. These dyes are known for their bright fluorescence and compatibility with various imaging techniques. However, they also present challenges such as poor solubility which can result in the formation of dye aggregates. These aggregates can be confused with actual EVs, complicating the detection and analysis process.
While lipophilic dyes like PKH and carbocyanine dyes are valuable tools for EV labelling, their limitations necessitate careful consideration and experimental design. Researchers must account for potential non-specific binding, aggregate formation, and impacts on membrane properties to ensure accurate and reliable EV detection. Exploring alternative dyes and labelling techniques may help mitigate these issues and enhance the precision of EV studies.
Innovative Membrane Dyes by Biotium: Elevating EV Labelling to New Heights
Maintaining the integrity of EVs while ensuring accurate fluorescent labelling is paramount for reliable analysis. Biotium has revolutionised EV labelling with their groundbreaking membrane dyes, offering a remarkable improvement over conventional methods.
ExoBrite™ True EV Membrane Stains
ExoBrite™ True EV Membrane Stains represent a breakthrough in EV labelling technology. These lipophilic dyes are meticulously designed to provide comprehensive coverage of EVs within a sample, ensuring near-complete labelling efficiency. Unlike traditional dyes such as PKH, DiO, DiI, and DiD, ExoBrite™ True EV Membrane Stains offer superior performance and compatibility with antibody co-staining techniques. By minimising background noise and maximizing signal intensity, these stains enhance the accuracy and reliability of EV analysis, empowering researchers to extract precise insights from their experiments.
ExoBrite™ EV Surface Stains
Biotium’s ExoBrite™ EV Surface Stains represent a paradigm shift in the detection of EV membrane surface markers. These fluorescent conjugates target specific surface proteins, including cholera toxin subunit B (CTB), wheat germ agglutinin (WGA), and Annexin V, with unparalleled precision. By minimizing aggregation compared to competitor dyes, ExoBrite™ EV Surface Stains ensure clear and distinct labelling of bead-bound EVs. This breakthrough technology facilitates the accurate identification and characterisation of EV populations, unlocking new possibilities for research in EV biology and beyond.
ExoBrite™ STORM CTB EV Stains
For researchers seeking advanced imaging capabilities, ExoBrite™ STORM CTB EV Stains offer unparalleled performance. These specialised fluorescent conjugates of CTB bind specifically to GM1 gangliosides on the surface of lipid rafts and EVs, enabling high-resolution STORM imaging. Incorporating STORM-validated CF® Dyes, these stains deliver exceptional clarity and sensitivity, even in the most demanding imaging conditions. Unlike traditional lipophilic dyes, ExoBrite™ STORM CTB EV Stains exhibit minimal background aggregation, ensuring precise identification and analysis of EVs with unprecedented accuracy.
The introduction of innovative dyes like ExoBrite™ by Biotium represents a significant leap forward in EV labelling and imaging technology. By overcoming the limitations of traditional dyes such as PKH and carbocyanine, these advancements provide researchers with reliable and precise tools for studying EV biology. These groundbreaking technologies not only enhance our understanding of EVs but also hold immense promise for their applications in medical research.
References:
Boudna, M., Campos, A.D., Vychytilova-Faltejskova, P. et al. Strategies for labelling of exogenous and endogenous extracellular vesicles and their application for in vitro and in vivo functional studies. Cell Commun Signal 22, 171 (2024). https://doi.org/10.1186/s12964-024-01548-3
Dehghani, M., Gulvin, S.M., Flax, J. et al. Systematic Evaluation of PKH Labelling on Extracellular Vesicle Size by Nanoparticle Tracking Analysis. Sci Rep 10, 9533 (2020). https://doi.org/10.1038/s41598-020-66434-7
Ma, N., Wu, C., Meng, Z. In vivo imaging and tracking of exosomes for theranostics. J. Innov. Opt. Health Sci. 2021; 14 (6) doi: 10.1142/S1793545821300056
Morales-Kastresana, A., Telford, B., Musich, T.A. et al. Labeling Extracellular Vesicles for Nanoscale Flow Cytometry. Sci Rep 7, 1878 (2017). https://doi.org/10.1038/s41598-017-01731-2
Nolan JP. Flow Cytometry of Extracellular Vesicles: Potential, Pitfalls, and Prospects. Curr Protoc Cytom. 2015;73:13.14.1-13.14.16. Published 2015 Jul 1. doi:10.1002/0471142956.cy1314s73
Pužar Dominkuš P, Stenovec M, Sitar S, et al. PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim Biophys Acta Biomembr. 2018;1860(6):1350-1361. doi:10.1016/j.bbamem.2018.03.013
Simonsen JB. Pitfalls associated with lipophilic fluorophore staining of extracellular vesicles for uptake studies. J Extracell Vesicles. 2019;8(1):1582237. Published 2019 Feb 20. doi:10.1080/20013078.2019.1582237
Zhou C, Cox-Vázquez SJ, Chia GWN, et al. Water-soluble extracellular vesicle probes based on conjugated oligoelectrolytes. Sci Adv. 2023;9(2):eade2996. doi:10.1126/sciadv.ade2996
CONTACT

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotaiton
OTHER BLOGS YOU MIGHT LIKE
HOW CAN WE HELP YOU? Our specialists are to help you find the best product for your application. We will be happy to help you find the right product for the job.

TALK TO A SPECIALIST
Contact our Customer Care, Sales & Scientific Assistance

EMAIL US
Consult and asked questions about our products & services

DOCUMENTATION
Documentation of Technical & Safety Data Sheet, Guides and more..