- Your cart is empty
- Continue Shopping
Plasmid DNA Production & Manufacturing Services
Atlantis Bioscience, the authorised partner of PackGene, provides researchers and biotech companies with comprehensive plasmid DNA production and manufacturing solutions — from research-grade plasmids to full GMP production.
Plasmids are produced by growing bacterial cells containing the desired DNA sequence, followed by extraction and purification. Through PackGene’s Plasmid Extraction service, Atlantis Bioscience delivers high-quality plasmids for transfection and preclinical research. With years of process optimisation, PackGene ensures consistent yield and purity — from 100 µg to 1000 mg and beyond, with high homogeneity and ultra-low endotoxin levels (<10 EU/mg) suitable for research and clinical applications.
Our integrated platform supports every stage of your workflow, from vector design to large-scale clinical production, ensuring purity, consistency, and regulatory compliance.
Service Overview
Plasmid Preparation
Plasmid Preparation
Flexible scales from lab use to preclinical and IND-grade DNA (CAMP) production
Research Grade Plasmid
Research Grade Plasmid
High-quality plasmids for transfection and assay development
Preclinical Plasmid DNA
Preclinical Plasmid DNA
Endotoxin-free, animal-free plasmids for IND-enabling studies
GMP Plasmid
GMP Plasmid
Fully GMP-compliant, DMF-registered plasmids for clinical and commercial use
Plasmid Preparation
Flexible scaling | Sequence fidelity | Rapid turnaround
PackGene’s plasmid preparation service supports lab-scale DNA production with guaranteed sequence accuracy and low endotoxin content.
Ideal for early-stage discovery, vector construction, and functional studies.
Features:
- Fast delivery for all drug development stages
- Flexible sizes from 100µg to 2g (preclinical) and up to 100g (GMP)
- Customizable QC (restriction enzyme digestion, sequencing, purity testing)
- Compatible with AAV, LNP, CAR-T, and vaccine applications
Key benefits
Service Details
QC standards
Flexible Sizing
We offer plasmid DNA production from 100 µg to 2 g for research and preclinical use, and up to 100 g for GMP manufacturing, supporting every stage from discovery to clinical production.
Precision
Our rigorous quality control system ensures accurate and reproducible plasmid DNA construction, maintaining sequence fidelity and structural integrity at all scales.
Speed
Optimised workflows enable industry-leading turnaround times with rapid and reliable delivery to accelerate your research progress.
Wide Compatibility
We provide plasmid grades suitable for all stages of drug development, from research and preclinical work to GMP manufacturing for clinical programmes.
Tailored QC Tests
Our customisable QC panel includes endotoxin testing, supercoiled content, residual impurities and sequence verification, ensuring data integrity and compliance.
Support for Various Projects
Our plasmids support a wide range of applications including cell transfection, protein expression, AAV packaging, mRNA production, vaccine and antibody development.
| Category | Research Grade | Preclinical Grade |
|---|---|---|
| Application | Molecular cloning, protein expression, transfection, viral packaging for research | Large-scale viral packaging, animal studies |
| Scale | 100 µg – 100 mg | 10 mg – 2 g |
| Timeline | As fast as 3 days | As fast as 2–3 weeks |
| Parameter | Research Grade | Preclinical Grade |
|---|---|---|
| Appearance | Clear, colourless, no visible particulates | Clear, colourless, no visible particulates |
| A260:280 | 1.8 – 2.0 | 1.8 – 2.0 |
| Homogeneity | Predominantly supercoiled | >90 ± 10% supercoiled |
| Restriction Analysis | Conforming to reference pattern | Conforming to reference pattern |
| Residual RNA | – | Undetected by SYBR Gold |
| Residual E. coli DNA | – | Quantitative PCR ≤ 1% |
| Endotoxin | ≤ 100 EU/mg or ≤ 10 EU/mg (optional LAL test*) | ≤ 10 EU/mg |
| Bioburden Testing | Optional* | No growth on agarose plate after 48 h |
| Sequencing | Optional* | GOI |
| Antibiotics-Free Production | No | Optional |
| Residual Host Protein | – | < 0.1% by HCP ELISA* |
| Animal-Free Production | – | TSE/BSE* |
| Mycoplasma Contamination | – | Quantitative PCR, Negative* |
| Material Archiving | Yes (plasmid only) | Yes (plasmid + bacteria) upon request* |
| pH | – | Potentiometry 7.5 – 8.0, USP <791>* |
| Kan | – | ELISA < 0.5 ng/g* |
| Sterility | – | USP <71>* |
| Osmolality | – | USP <785>* |
| Advanced Endotoxin Removal | – | < 0.005 EU/µg (available with additional charge) |
| Dedicated Purification Resin | – | Upon request* |
| Buffer | ddH₂O, TE or customer defined | ddH₂O, TE or customer defined |
| Dispensing | Upon request | Upon request |
| Dedicated Filtration Membrane | – | Sterile filtration* |
*Tests marked require additional charges and lead time.
Research Grade Plasmid Preparation
High-quality plasmids for discovery research and preclinical validation
Produced under stringent quality control, research-grade plasmids are ideal for in vitro transfection, viral vector packaging, and early functional studies.
Features:
- Flexible scale from 10 µg to 1000 mg with high homogeneity
- Low endotoxin levels for biosafety
- Suitable for transfection, viral production, CAR-T, and more
Key benefits
Service Details
QC standards
Flexible Sizing
Production scales from 100 µg to 2 g for preclinical use and up to 100 g for GMP manufacturing.
Sequence Fidelity Ensured
Each plasmid undergoes customisable QC testing to verify sequence accuracy.
Fast Delivery
Rapid turnaround for every development stage.
| Scale | Timeline (Business Days) |
|---|---|
| 100 µg | 3–5 |
| 200 µg | 3–5 |
| 500 µg | 3–5 |
| 1 mg | 5–7 |
| 2 mg | 5–7 |
| 10 mg | 8–10 |
| 20 mg | 8–10 |
| 50 mg | 8–10 |
| Parameter | Standard | Analytical Method |
|---|---|---|
| Homogeneity | Predominantly supercoiled | Densitometry upon agarose gel electrophoresis |
| Appearance | Clear, no visible particles | Visual inspection |
| A260/280 | 1.80 – 2.00 | Nanodrop UV absorption |
| Restriction Analysis | Conforms to reference | Enzyme digestion and electrophoresis |
| Endotoxin | <100 EU/mg or <10 EU/mg | Spot check |
Preclinical Plasmid DNA
Bridging discovery to IND submission
Preclinical-grade plasmids are manufactured with enhanced traceability, animal-free production, and comprehensive quality documentation suitable for regulatory submissions.
Highlights:
- Endotoxin-free plasmids with optional animal-free processing
- Suitable for in vivo studies from mice to NHP
- Available in quantities from 10 mg up to 100g
Key benefits
Production Details
QC standards
Comprehensive quality control of supercoiled content and endotoxin levels.
Quantitative impurity analysis for reliable plasmid assessment.
Animal-free production option available.
Guaranteed quantity of mg to gram with material archiving option
| Process Step | Preclinical |
|---|---|
| Clone screening | √ |
| Growth condition optimisation | √ |
| High-density fermentation | √ |
| Alkaline lysis | √ |
| Chromatographic purification | √ |
| Antibiotics-free | √ |
| Consistent manufacturing process | √ |
| Instrument calibration / validation | Calibration |
| COA & CoC | COA |
| TSE / BSE | O |
| Antibiotics-free production | √ |
| Animal-free production* | O |
| Material archiving | Plasmid archived, additional charges for bacteria |
| Test | Method |
|---|---|
| Appearance | Visual inspection |
| A260/280 | Ultraviolet-visible spectrophotometry (A260/A280) |
| Homogeneity | HPLC |
| Restriction Analysis | Agarose gel |
| Residual RNA | SYBRGold |
| Residual E. coli DNA | qPCR |
| Endotoxin | LAL |
| Bioburden Testing | Direct inoculation |
| Sequencing | Sanger |
| Residual Host Protein | HCP ELISA |
| Mycoplasma Contamination | qPCR |
| pH | Potentiometry |
| Residual Kanamycin | ELISA |
| Sterility | |
| Osmolality |
GMP Plasmid
Clinical-grade, DMF-registered plasmid DNA for global regulatory submission
PackGene’s GMP plasmid manufacturing adheres to ISO 13485 and GMP standards and is Drug Master File (DMF) registered, providing global regulatory support for gene and cell therapy programs.
Key Features:
- cGMP plasmid production up to 200L with flexible scales
- Strict contamination control using isolated lines and single-use tech
- Complete batch documentation and CoA provided
- Residual host DNA, protein, sterility, and identity testing included
- Suitable for IND filing, clinical trials, and commercial manufacturing
- Over 10 years of expert manufacturing with full process support
Key benefits
Service Details
Performance
Productivity
Two independent cGMP production lines supporting up to 200 L production scale.
Quality
Isolated production lines, rigorous cleaning procedures, and disposable materials ensure complete prevention of cross-contamination.
Flexibility
Multiple production scales and grades enable high-efficiency project completion at a reasonable cost.
Expertise
PackGene’s experienced production team brings over 10 years of expertise in plasmid manufacturing.
Advanced Equipment
Single-use technology is applied across upstream and downstream processes, with Fill & Finish performed under VHP isolator conditions.
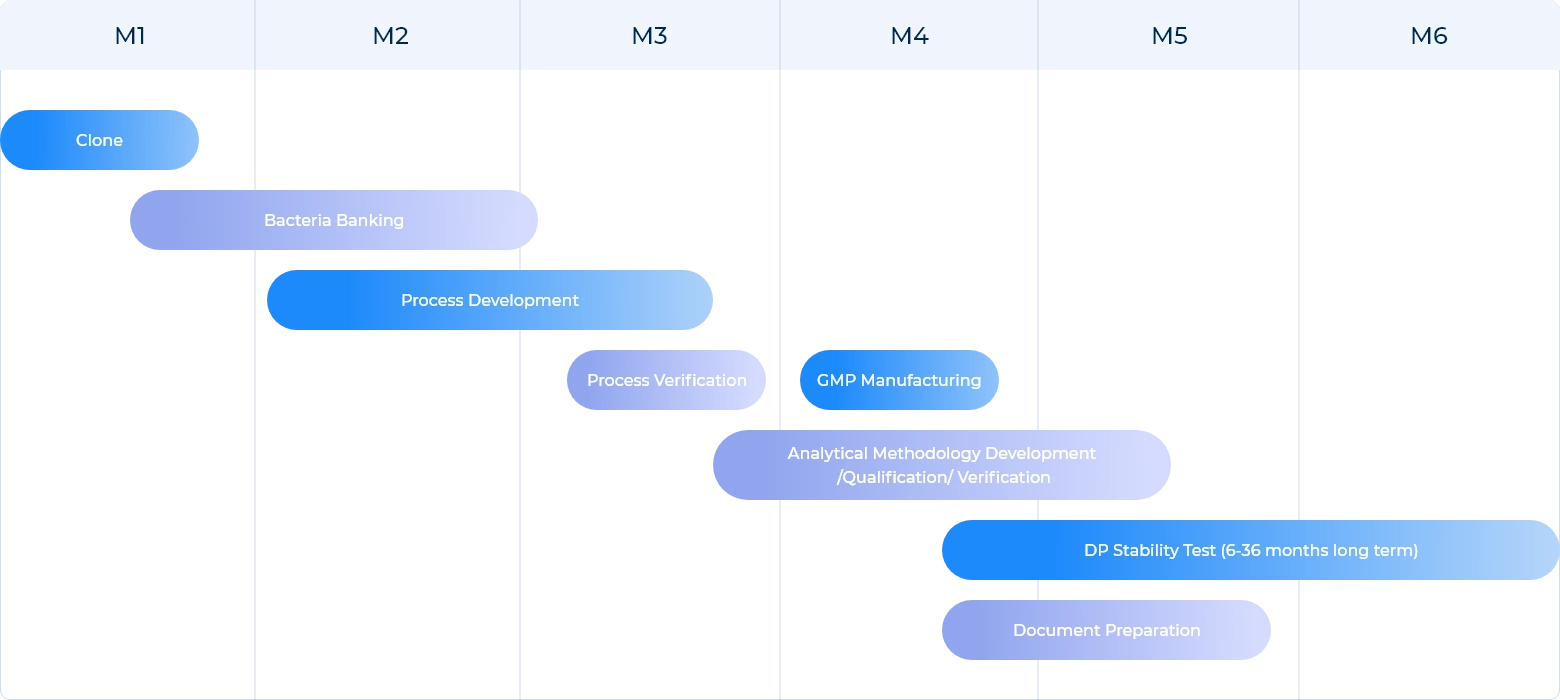
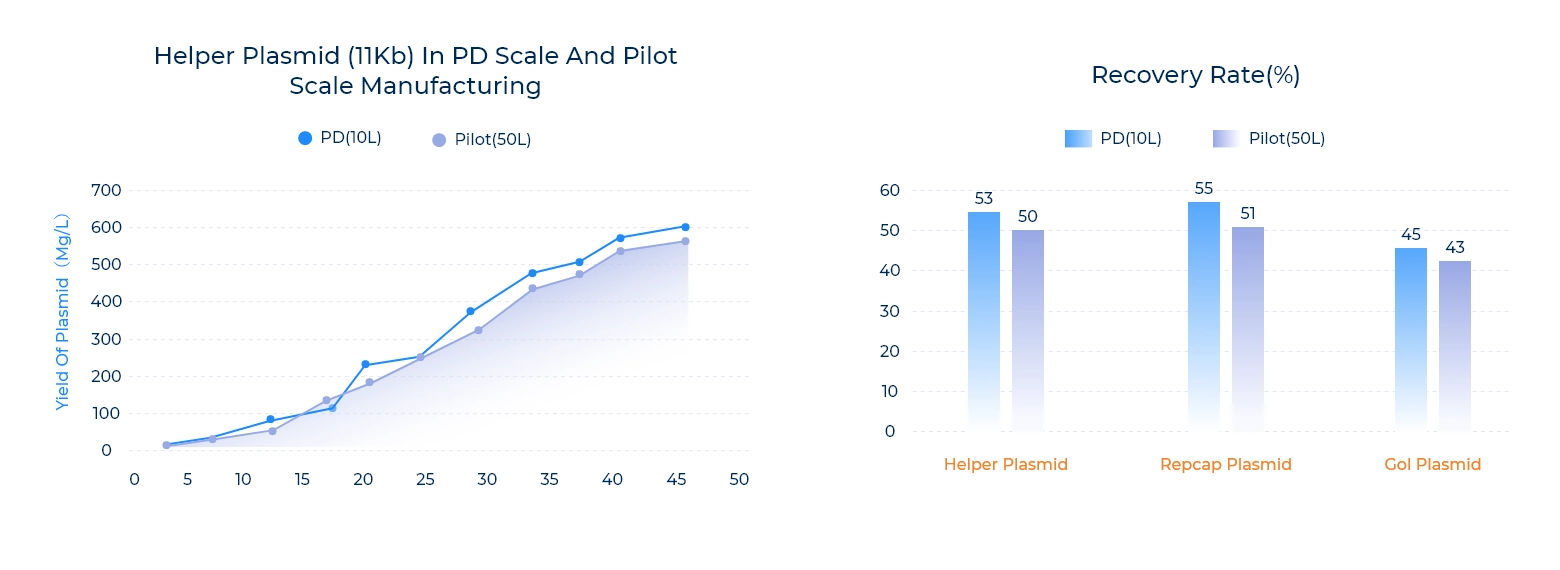
Scalability
Our efforts to optimize the plasmid prep process have resulted in increased efficiency and scalability that drive consistent batch to batch yields with regard to both fermentation and purification recovery rates.
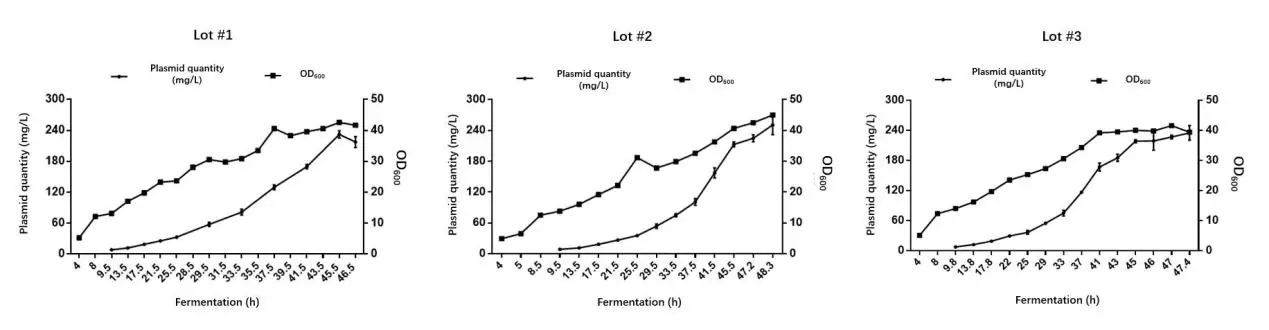
Batch-to-batch consistency
The examples below demonstrate our fermentation yield consistency across three separate fermentation rounds using the same AAV cis plasmid template in a 50L fermenter.
State-of-the-Art Plasmid DNA Manufacturing Facility

As the authorised partner of PackGene, Atlantis Bioscience provides access to world-class plasmid DNA production and GMP manufacturing facilities that meet the highest global quality standards.
The 11,000-square-foot GMP facility, equipped with 5 L to 200 L fermenters, supports both process development (PD) and large-scale GMP plasmid production. With an annual capacity exceeding 1,000 g of GMP-grade plasmid DNA, PackGene enables reliable, high-yield manufacturing for AAV vector, mRNA vaccine, and gene therapy applications.
A dedicated team of over 40 experts with decades of experience in AAV plasmid preparation ensures round-the-clock operations and consistent quality. Together, Atlantis Bioscience and PackGene empower researchers in Singapore and Southeast Asia to accelerate translational research from bench to bedside.
Applications of Plasmid DNA Manufacturing
Our high-quality plasmid DNA supports a wide range of biomedical and translational research applications, providing reliable performance across discovery, preclinical, and GMP stages.
Key applications include:
- AAV and LNP vector manufacturing for efficient gene delivery and therapeutic development
- mRNA template generation for vaccine and RNA-based drug production
- CAR-T and gene therapy manufacturing, enabling consistent and scalable plasmid supply
- CRISPR/Cas9 delivery systems for precise genome editing research
- Vaccine and antibody development, from early R&D to large-scale validation
Real-World Example of Translational Support
At Atlantis Bioscience, we bridge research and clinical application by supporting translational teams with GMP-like plasmid sourcing, mRNA, and LNP services. We recently helped a spin-off from a leading research institution obtain a GMP-like plasmid for their clinical trial application, ensuring quality, compliance, and timely delivery.
Through our partnership with PackGene Biotech, a global CRO and CDMO specialising in AAV vectors, mRNA, plasmid DNA, and lentiviral solutions, we provide integrated support across drug delivery, vaccine development, and viral vector manufacturing. From early discovery to cell and gene therapy trials, we enable seamless translation from bench to bedside.
Partner with Atlantis Bioscience

Atlantis Bioscience proudly serves as PackGene’s authorized partner, bridging global plasmid manufacturing excellence with local research expertise.
We support academic, biotech, and translational teams through every stage — from small-scale plasmid prep to GMP-grade, DMF-registered manufacturing.
Get in touch to discuss your plasmid production needs or request a quotation tailored to your workflow.
Want To Inquire About The Services?
Contact Us

THE ATLANTIS BIOSCIENCE DIFFERENCE Discover Translational Solutions To Advance From Bench to Bed
GET SUPPORT Whenever You Need It

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotation.





